Abstract
Ig heavy chain class switch recombination (CSR) involves a recombination/deletion mechanism that exchanges the expressed CH gene with a downstream CH gene. CSR is mediated by highly repetitive switch (S) region sequences and requires the activation-induced deaminase (AID). The S region 5′ of the Cμ gene (Sμ) can undergo high-frequency internal deletions in normal B cells and B cell lines activated for CSR, although the relationship of these deletions and CSR has not been elucidated. In this study, we introduced constitutively transcribed Sμ or Sγ2b regions into a pro-B cell line that can be activated for AID expression, CSR, and endogenous Sμ deletions. We find that randomly integrated S region transcription units in these cells also undergo increased levels of internal rearrangements after cellular activation, indicating that the deletion process is independent of location within the Ig heavy chain locus and potentially AID-promoted. To test the latter issue, we generated hybridomas from wild-type and AID-deficient activated B cells and assayed them for internal Sμ deletions and S region mutations. These studies demonstrated that efficient intra-S region recombination depends on AID expression and that internal S region deletions are accompanied by frequent mutations, indicating that most S region deletions occur by the same mechanism as CSR.
The genes that encode the Ig heavy chain (IgH) and light chain (IgL) variable regions are assembled from component V, D, and J segments during early B cell development by the site-specific V(D)J recombination reaction (reviewed in ref. 1). Subsequently, in activated mature B cells, the exons that encode the first IgH constant region expressed during development (Cμ) can be replaced with one of a set of downstream CH exons via a process referred to as IgH class switch recombination (CSR). CSR allows the CH-encoded effector portion of an Ig molecule to be replaced, while preserving the antigen-binding variable region (reviewed in ref. 2). CSR involves a recombination/deletion process that generally occurs between large, repetitive switch (S) region sequences that lie just upstream to all CH exons that undergo CSR. In this context, the CH exons are organized 5′-V(D)J-SμCμ-Cδ-Sγ3Cγ3-Sγ1Cγ1-Sγ2bCγ2b-Sγ3Cγ3-SɛCɛ-SαCα-3′. Thus, switching from IgM to another IgH isotype involves a recombination event between the Sμ and downstream S regions with deletion of all intervening DNA. S regions range from 1 to 10 kb in length and consist of repetitive units based on pentamers (Sμ, Sɛ, and Sα) or 49-bp repeats (Sγ3, Sγ1, Sγ2b, and Sγ2a). With respect to given S regions, CSR junctions occur throughout the S region and occasionally in sequences just outside (3, 4). An additional genomic alteration process in mature B cells, referred to as somatic mutation (SM), introduces mutations into the IgH and IgL variable regions in the context of an antigenic response (reviewed in refs. 5 and 6).
CSR can be induced in vitro by stimulating splenic B cells with an activator [e.g., anti-CD40 or lipopolysaccharide (LPS)] in the presence of specific cytokines, which mimic in vivo T-dependent and T-independent pathways. The type of stimulatory treatment directs CSR to specific downstream S regions via a mechanism that involves the specific induction of germ-line CH transcription (reviewed in refs. 2 and 7). In this regard, all CH genes are organized into units in which transcripts initiate from a promoter 5′ of the S region, run through the S region, and are polyadenylated downstream of the CH exons. Various lines of evidence indicate that germ-line transcription per se plays a direct role in guiding CSR (reviewed in refs. 2 and 7). Such a role may either involve making transcribed S regions “accessible” to factors that initiate CSR or functioning directly in the CSR mechanism. Activation of B cells also induces the expression of activation-induced deaminase (AID), a protein absolutely required for CSR, as well as SM (8, 9). Mice deficient for AID have slightly elevated serum IgM but no other detectable IgH isotypes (8). A defect in AID-deficient B cell activation was ruled out by demonstrating that germ-line transcription was initiated normally and that germinal center formation was intact. AID appears to be the only lymphoctye-specific factor required for CSR as its expression can induce CSR in appropriate substrates in nonlymphoid cells (10). Various functions have been proposed for AID based on its homology to RNA-editing proteins; although its precise function remains unknown (reviewed in refs. 2 and 11).
For CSR, the DNA of the involved S regions must be first broken and then rejoined. Some studies have suggested enzymatic activities specific for a given S region, whereas others have argued for more generalized activities secondary to S region transcription (reviewed in refs. 2 and 11). Notably, both double-strand breaks and staggered single-strand breaks have been implicated as early intermediates in the CSR process (12, 13). Once initiated, CSR has been shown to require an intact nonhomologous DNA end-joining system for completion (reviewed in ref. 2). Recent studies have suggested that AID may function to induce S region-specific DNA lesions that precede CSR (14, 15). In this context, histone H2AX is phosphorylated soon after DNA damage, and phosphoryalated H2AX (γ-H2AX) can be detected in nuclear foci thought to represent sites of DNA breaks (16). γ-H2AX foci colocalize with IgH loci in wild-type (WT) B cells activated for CSR, but not similarly activated AID-deficient B cells (14), implying that CSR-induced DNA breaks do not occur in the absence of AID. Frequent mutations were observed in regions flanking CSR junctions in normal B cells, suggesting that joining in CSR is affected by an error-prone DNA repair mechanism (3, 17). On the other hand, recent studies showed that sequences immediately upstream of the core Sμ repeats have a high level of mutations in B cells activated for, but that have not yet undergone, CSR. As these mutations were not observed in activated AID-deficient B cells, these findings were interpreted to mean that AID is responsible for generating DNA breaks before CSR (14, 15). These studies also implied that initiation of CSR might involve multiple lesions in the Sμ region with their resolution via CSR requiring similar lesions in a participating downstream S region.
The 18–8 Ableson murine leukemia virus-transformed cell line undergoes CSR at high frequency (18, 19) and also accumulates a high level of internal Sμ deletions during culture (18), suggesting that the two processes may be related. Likewise, additional studies documented both internal Sμ deletions in hybridomas, as well as deletions in downstream S regions in B cells activated for CSR but in which Sμ was inactivated for S region recombination (20–23). Although the exact relationship to internal S region deletions and CSR is not clearly understood, the final outcome of S region lesions, CSR or internal deletion, might be in part affected by the relative availability of donor and recipient S regions. In this study, we have further characterized the internal S region deletion phenomenon and addressed its relationship to bona fide CSR.
Methods
DNA Constructs and Gene-Targeted Replacement of AID.
A 6-kb BamH1 fragment containing Iγ2b-Sγ2b was cloned from pKEO (24) into the expression construct pEμ (25) to generate the Dγ2b substrate. To make μGFP, a 7.5-kb EcoRI/BamHI fragment containing Iμ-Sμ−Cμ (exons 1 and 2) was cloned into pBR322 vector. The simian virus 40 polyadenylation signal was PCR-amplified from pcDNA1Amp (Invitrogen) and ligated into the Xho site at the 3′ end of pcgEGFP and then subsequently blunted to the first exon of Cμ. Finally, a XbaI/EcoRI 0.7-kb Eμ genomic fragment was blunted to the 5′ end of the Iμ region. The targeting construct to disrupt the AID gene in TC-1 embryonic stem cells consists of a 9-kb XhoI fragment containing exon 1 as a 5′ arm and a 4-kb SmaI/PstI fragment containing part of exon 3 as a 3′ arm, cloned into the pLNTK construct. After selection, a clone was confirmed to delete exon 2 and part of exon 3 of the AID gene by Southern blotting and doubly deficient cells were assayed via Rag-2−/− blastocyst complementation. Chimeric animals were analyzed at 6–12 weeks of age.
Cell Culture, FACS Analysis, and Generation of Hybridomas.
The 18–81, A20, and NS-1 lines have been described (18, 26). Splenic B cells were cultured, stimulated, and analyzed by FACS as described. To generate hybridomas, activated B cells from day 4 LPS-, LPS + IL-4-, or anti-CD40 + IL-4-treated cultures were fused to the NS-1 cells, and fusion lines were screened for IgM or IgG secretion by ELISA using isotype-specific antibodies (SBA). Clonal status and IgH allele numbers were determined by Southern blotting using a JH-specific probe on Sac1 or EcoR1-digested DNA (23).
Hybridoma Sequence Analysis.
Hybridoma DNA was isolated and used in a PCR with Pfu polymerase (Stratagene). The sequence of the forward primer is CTGGACTCAACTGGGCTGGCTGATGGGATG and of the reverse primer is CCAGCCCAGCAGCAGGCCAGTTCAACACAT. A product of the predicted size (301 bp) was cloned into the Topo-TA (Invitrogen) plasmid and sequenced. Sequences were analyzed by using dnastar software and aligned by using the corresponding genomic sequence from the Celera database, as well as sequences obtained from TC-1 embryonic stem cell genomic DNA.
DNA and RNA Hybridization and Probe Preparation.
Isolation of DNA, RNA, and blotting was done as described (23, 27). Probes for the IgH locus have been described (18, 20). The human β-globin probe was isolated as a 0.9-kb ApaI/EcoR1 restriction fragment including portions of exons 2 and 3. Green fluorescent protein (GFP) cDNA was used as a probe to detect the μGFP substrate. A 1.4-kb HindIII/PstI fragment that includes exon 3 of the AID gene was used as a 3′ probe for Southern blot analysis to detect targeted disruption of the AID gene. The complete AID cDNA was used as a Northern probe.
Results
Introduction of Intra-S Recombination Substrates into AID-Expressing Pro-B Lines.
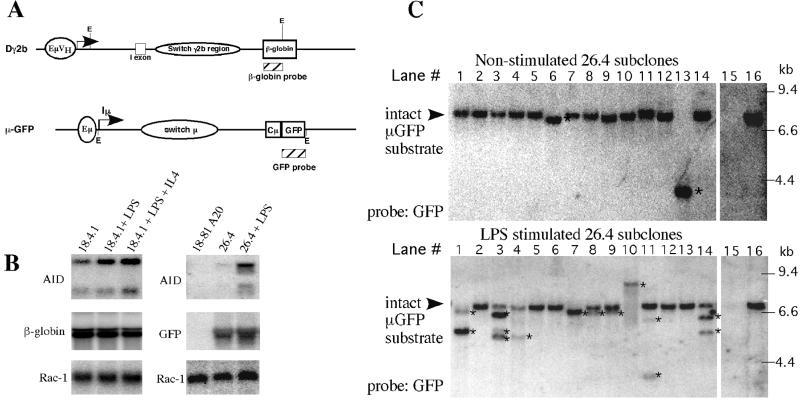
To further analyze the S region deletion phenomenon, we generated internal S region recombination (ISR) substrates consisting of S region transcription units in combination with the intronic IgH transcriptional enhancer (iEμ) and VH or I promoters (Fig. 1A). The Dγ2b construct contains the iEμ in combination with the 186.2 VH promoter, driving expression of Iγ2b-Sγ2b sequences. A genomic DNA fragment from the human β-globin locus is located downstream of the Sγ2b region and allows specific detection of the substrate by Southern and Northern blotting analyses. The μ-GFP construct contains iEμ−Iμ−Sμ through the first Cμ exon, followed by GFP coding sequence that can be used as a probe (Fig. 1A). The general design allows specific detection of recombination events within the substrate, while preserving similar organization to the genomic configuration of the endogenous locus with respect to proximal regulatory elements and splice donor and acceptor sites. For assays, we used the 18–8 pro-B line that undergoes CSR to Cγ2b and also shows a very high level of internal Sμ deletions (18). The 18–8A20 line (A20) is a subclone of 18–8 that has deleted both endogenous Sμ regions (18). Northern analysis confirmed that expression of AID in both 18–8 and A20 cells increased after treatment of the cells with LPS or LPS + IL-4 (Fig. 1B). Notably, however, we observed significant levels of AID transcripts in untreated 18–8 (28), consistent with its ability to undergo spontaneous CSR and internal Sμ deletions (18). In contrast, we observed much lower constitutive AID levels in the A20 subclone, but induced levels were similar to those of 18–8 (Fig. 1B).
Figure 1.
Induction of intra-S region deletions detected in transfected ISR substrates. (A) Design of the Dγ2b and μ-GFP constructs showing the position of S region sequences in relation to restriction sites and hybridization probes (hatched boxes) used for Southern analysis. See text for details. E, EcoR1. (B) (Left) AID transcripts are detectable by Northern blotting in the Dγ2b-containing subclone 18.4.1 and increase after activation. Dγ2b transcripts as detected by the β-globin probe display constitutive expression from the substrate even after activation. Rac-1 levels were used to determine RNA loading (33). (Right) AID transcripts are induced in μ-GFP subclone 26.4 after activation with LPS. (Middle) A Northern blot with GFP probe showing constitutive expression of the μ-GFP construct in subclone 26.4. (Bottom) A Northern blot of the same RNA hybridized with a Rac-1 control probe to indicate RNA loading. (C) Deletions within the Sμ region of the μ-GFP construct occur after activation of 18–81 A20 cells with LPS. Southern blotting analysis on DNA from 18–81 A20 (lane 15), 26.4 (lane 16), and 26.4 subclones (lanes 1–14). μ-GFP transfected (26.4) 18–81 A20 cells were grown in LPS or media alone for 2 days and then subcloned at limiting dilution. Genomic DNA was isolated and digested with EcoR1 and screened with the GFP-specific probe shown in A. Rearrangements are denoted by *.
Internal Deletion of ISR Substrates Correlates with AID Expression in Pro-B Lines.
We stably transfected the Dγ2b and μGFP constructs into the 18–8 or A20 cell lines and assayed for subclones with intact integrations by Southern blotting by using an EcoR1 digest and the human β-globin- (Dγ2b) or GFP-specific probes (μ-GFP) (Fig. 1C). We then confirmed by Northern blotting that subclones appropriately transcribed the constructs (Fig. 1B). Subclone 18.4.1, which contains 2–3 intact copies of Dγ2b randomly integrated into the 18–8 cell line, and subclone 26.4, which contains a single intact copy of μ-GFP randomly integrated into 18–81 A20 cells, were selected for in-depth analyses (Table 1, Fig. 1C, and Fig. 4, which is published as supporting information on the PNAS web site, www.pnas.org). Subclone 18.4.1 was grown for 48 h in the presence of LPS, LPS + IL-4, or media alone and then subcloned. Southern analyses revealed that approximately 10% of the nonstimulated 18.4.1 subclones had rearrangements of Sγ2b sequences, detectable as one or more bands of a different (usually smaller) size than the intact integration often with diminution of the intensity of the intact band (Table 1 and Fig. 4). Cellular activation with LPS and LPS + IL-4 led to a modest increase in the frequency of subclones with rearrangements to 14% and 20%, respectively (Table 1 and Fig. 4). In a second experiment, 18% subclones from nonstimulated and 24% from LPS + IL-4-stimulated cultures had detectable intra-Sγ2b deletions (Table 1 and Fig. 4). Similar results were observed with several other 18–8 transfectants; construct deletions were detected in all; however, the frequency varied among transfectants and after cellular activation (data not shown). Thus, cellular activation generally correlated with the recovery of an increased number of subclones with substrate deletions within the Dγ2b or related γ2b substrates in 18–8 cells, but a substantial level of deletion occurred without stimulation. In this regard, we note that the 18–8 cell line has significant basal levels of AID transcripts (Fig. 1B), and subclones vary in their induction level. Therefore, although specific deletions were observed, we were not able to clearly correlate their relationship to activation and AID expression based on these analyses.
Table 1.
In vitro stimulation of pro-B cell lines induces deletions in substrate S sequences
| Substrate/clone # | Stimulation | Deletions/subclones |
|---|---|---|
| Dγ2b/18.4.1 Exp. #1 | Nonstimulated | 6/61 (9.8%) |
| Dγ2b/18.4.1 Exp. #1 | LPS | 6/42 (14.3%) |
| Dγ2b/18.4.1 Exp. #1 | LPS + IL-4 | 8/41 (19.5%) |
| Dγ2b/18.4.1 Exp. #2 | Nonstimulated | 6/34 (17.6%) |
| Dγ2b/18.4.1 Exp. #2 | LPS + IL-4 | 10/42 (23.8%) |
| μ-GFP/26.4 Exp. #1 | Nonstimulated | 5/67 (7.5%) |
| μ-GFP/26.4 Exp. #1 | LPS | 14/62 (22.6%) |
| μ-GFP/26.4 Exp. #2 | Nonstimulated | 8/44 (18.2%) |
| μ-GFP/26.4 Exp. #2 | LPS | 12/23 (52.2%) |
To demonstrate that ISR is not limited to Sγ2b sequences or the Dγ2b substrate and to use a line with a lower background of AID, we tested subclones of A20 cells transfected with the μ-GFP construct. A20 cells were used because they have no endogenous Sμ sequences and also because they have much lower basal levels of AID expression. Subclone 26.4 containing a single copy of the μ-GFP construct in the A20 line was grown for 2 days in LPS-containing media, or media alone, and then subcloned by limiting dilution. EcoR1-digested genomic DNA was screened by Southern blot analysis for ISR with a GFP-specific probe (Fig. 1C). In two experiments, 5/67 (7.5%) or 8/44 (18.2%) of the subclones isolated from the nonstimulated culture had evidence of deletions in the EcoR1 fragment flanking Sμ (e.g., Fig. 1C Upper, lanes 6 and 13). In comparison, 14/62 (22.6%) or 12/23 (52%) of the subclones isolated after 2 days of LPS stimulation had detectable ISR of Sμ sequences within the μ-GFP construct (e.g., Fig. 1C Lower, lanes 1, 3, 4, 7–11, and 14). In the subclones from the LPS-treated lines, multiple rearranged bands frequently were detected indicating ongoing rearrangement after treatment (Fig. 1C; rearrangements denoted with *). As a confirmatory probe, Sμ was also used to probe Southern blots and demonstrated rearrangement within the 5.5-kb Xba1 construct fragment (data not shown). In another μ-GFP-transfected clone, 38.1, the number of subclones with Sμ ISR did not alter with LPS stimulation. However, Northern blot analysis revealed that AID expression was not induced after LPS treatment in this subclone (data not shown), supporting the notion that AID is required for this process. Thus, ISR occurs at a high level in randomly intergrated S region constructs in pro-B cell lines. Moreover, activation of these cells enhances ISR in an Sμ-containing construct randomly integrated into the genome and appears to correlate with the induction of AID expression; although induction variability makes statistical analysis of this correlation difficult.
Hybridomas from AID-Deficient B Cells Contain Fewer Intra-Sμ Rearrangements than Do WT IgM-Secreting Hybridomas.
To test the role of AID on intra-Sμ region deletions in normal B cells, we generated embryonic stem cells with the AID gene disrupted on both alleles, resulting in a similar targeted deletion as described (8) (Fig. 5, which is published as supporting information on the PNAS web site). As expected, AID−/− lymphocytes secreted only IgM in the serum of chimeric mice, and analysis of Ig levels in supernatants of stimulated AID−/− splenic B cells revealed the expected lack of CSR after appropriate stimulation (Fig. 5 and data not shown). To facilitate assessment of the role of AID in intra-Sμ deletions, we generated hybridomas via fusion of the NS-1 line with WT and AID−/− B cells after stimulation in vitro for 4 days with LPS, LPS + IL-4, and anti-CD40 + IL-4. ELISA analyses confirmed that all hybridomas generated from AID−/− B cells (referred to as AID−/−-derived hybridomas) secreted IgM and no other isotypes (three independent fusions, 272 total hybridoma lines, data not shown). In comparison, 98/353 (28%) of hybridomas generated from three independent fusions of WT B cells (WT hybridomas) secreted one of the IgG isotypes and not IgM (data not shown).
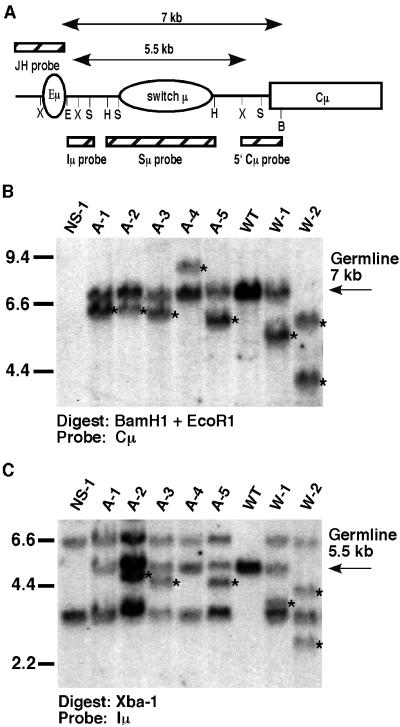
We assessed for the number of JH-containing alleles, Sμ rearrangements, and CSR in the hybridomas by using multiple specific probes and digests as described (Fig. 2) (23, 27). Representative data are shown for hybridomas (Fig. 2 B and C) by Southern blot assay of BamH1 + EcoR1 or Xba1-digested genomic DNA with a Cμ or Iμ probe (respectively). We found that 15 of 117 (12.8%) IgM-secreting WT hybridomas displayed evidence of novel Sμ rearrangements that did not involve CSR to a downstream S region (referred to as Sμ deletions) (Fig. 2; Table 2). Of these 15 WT hybridomas, 13 had two JH-containing alleles; of these, six had one Sμ-deleted allele and one germ-line Sμ allele, three had one Sμ-deleted allele and one switched allele (nonproductive allele), and four had Sμ deletions on both alleles (Fig. 2 B and C; Table 2). Two of the 15 WT hybridomas harbored only one nonfusion partner-derived JH-hybridizing allele that contained an Sμ deletion.
Figure 2.
Decreased frequency of Sμ deletions in hybridomas derived from AID−/− B cells compared with WT, IgM-secreting hybridomas. (A) Genomic organization of the JH-Cμ region of the IgH locus, including the location of the intronic IgH enhancer (Eμ), μS region (Sμ), and μ constant region exons (Cμ). B, BamH1; E, EcoR1; H, HindIII; S, Sac1; X, XbaI. Two-sided arrows indicate germ-line EcoR1/BamH1 and Xba1 restriction fragment sizes. Hatched boxes represent hybridization probes used for Southern blotting analyses. (B) Panel of hybridomas showing internal deletions within Sμ. Hybridoma DNA was doubly digested with BamH1/EcoR1 and Southern analysis was performed by using the 5′ Cμ−specific probe shown in A. W1 and W2 are two representative WT IgM-secreting hybridomas; A1-A5 are AID−/− hybridomas. NS-1 is the hybridoma fusion partner. Kidney DNA (WT) indicates germ-line configuration. (C) To confirm that the deletions were between the Eμ and Cμ regions, Southern blotting analysis was done on Xba1-digested DNA and probed with the Iμ probe shown in A. Results on all Sμ-deleting hybridomas are summarized in Table 2. Rearrangements are denoted by *.
Table 2.
Sμ deletions in WT and AID−/− hybridomas
| Probe/digest
|
||||
|---|---|---|---|---|
| JH/S or E | Iμ/X | Sμ/X | Cμ/B+E | |
| WT IgM+ hybridomas with Sμ rearranged: 15/117 (12.8%) | ||||
| W-1 | R/R | G/R | G/R | G/R |
| W-2 | −/R | R/R | −/R | R/R |
| W-3* | −/R | −/− | −/R | −/R |
| W-4 | R/R | G/R | G/R | G/R |
| W-5 | R/R | R/R | R/R | R/R |
| W-6 | R/R | R/R | R/R | R/R |
| W-7 | R/R | G/R | G/R | G/R |
| W-8 | R/R | G/R | G/R | G/R |
| W-9 | −/R | nd | −/R | −/R |
| W-10 | R/R | nd | G/R | G/R |
| W-11 | R/R | nd | R/R | R/R |
| W-12 | R/R | nd | G/R | G/R |
| W-13 | R/R | nd | −/R | −/R |
| W-14 | R/R | nd | −/− | −/R |
| W-15 | R/R | nd | −/R | −/R |
| WT controls | ||||
| C-1 (IgG+) | R/R | −/− | −/− | −/− |
| C-2 (IgM+) | R/R | nd | G | G |
| AID−/− IgM+ hybridomas with Sμ rearranged: 5/214 (2.3%) | ||||
| A-1† | −/R | G | G/R | G/R |
| A-2 | R/R | G/R | G/R | G/R |
| A-3 | R/R | G/R | G/R | G/R |
| A-4† | −/R | G | G/R | G/R |
| A-5 | −/R | G/R | G/R | G/R |
nd, not done; G, germ line; R, rearranged; −, undetectable or comigrating band; B, BamH1; E, EcoR1; H, HindIII; S, Sac1; X, Xba1.
A rearranged Iμ band is detectable by using a BamH1 + EcoR1 double digest.
Sμ rearrangements appear to be the results of aberrant V(D)J joining.
In contrast to the WT hybridomas, only five of 214 (2.3%) AID−/−-derived hybridomas had detectable Sμ deletions, a highly significant difference (P = 0.0001 by Fisher's Exact test; Fig. 2, Table 2, and data not shown). Of these, in addition to the fusion partner alleles, two had two detectable JH-hybridizing alleles (A2 and A3) and the other three (A1, A4, and A5) had only one novel JH-hybridizing allele (Fig. 2, Table 2). Hybridization with an Iμ probe revealed two novel rearrangements in A5, indicating that the absence of two novel JH-hybridizing alleles most likely results from comigrating bands, underscoring the importance of using multiple probes and digests in these analyses. Therefore, we can conclude that A2, A3, and A5 clearly have intra-Sμ deletions. However, an Iμ probe failed to detect two alleles for clones A1 and A4, making it impossible to distinguish between two possibilities: either these alleles were products of an aberrant V(D)J rearrangement with the deletion spanning the JH, Eμ, and Iμ regions, or DNA lesions related to Sμ recombination were initiated upstream of the core Sμ region (as reported in ref. 29), with subsequent deletion of the Iμ and JH sequences. In any case, we conclude that Sμ deletion can be detected in AID−/−-derived hybridomas although at greatly reduced frequency.
Increased Frequency of Mutations 3′ of Sμ in WT B Cell Hybridomas That Have Undergone Intra-Sμ Sequence Deletions.
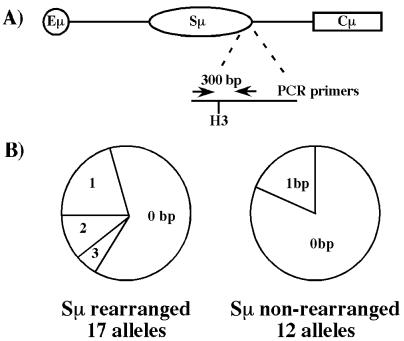
To further establish the relationship between intra-Sμ deletions and CSR, we sought to examine the Sμ region for evidence of mutations, using the IgM-secreting hybridomas with known CSR and Sμ deletion status. Although previous studies only examined the 5′ portion of the Sμ region, we focused on the 3′ portion of Sμ to avoid amplifying the NS-1-derived sequence, which still harbors the region 5′ of the core Sμ subsequent to CSR events. Therefore, we sequenced a 301-bp region 3′ of the repeat region of Sμ in selected IgM-secreting hybridomas. Mutations were detected in sequences from six of nine hybridomas, in which 12 of 17 B cell-derived alleles harbored an Sμ deletion. Seven of 17 (41%) sequenced alleles had alterations from the germ-line sequence (Fig. 3B and Table 3, which is published as supporting information on the PNAS web site), with a mutation frequency of about 1/500 bp. Four of the seven mutated alleles could be correlated to an upstream internal Sμ deletion as detected by Southern analysis (Fig. 2 B and C and data not shown). In contrast, 10 of 12 sequenced alleles from WT hybridomas with no detectable Sμ deletions by Southern blotting had no mutations, whereas the other two had only a single mutation (Table 3). These findings demonstrate mutations downstream of the core Sμ sequence in the absence of CSR, and moreover, suggest that they usually are associated with a detectable Sμ deletion. No mutations were detected in 16 alleles sequenced from eight tested AID−/−-derived hybridomas, including those that had undergone a deletion within Sμ.
Figure 3.
Mutations accumulate downstream of Sμ in WT IgM-secreting hybridomas. (A) The genomic configuration of the Sμ region is shown. PCR primers were designed 300 bp apart at the 3′ end of the Sμ core and used to amplify this sequence from hybridoma DNA. (B) (Left) The number of mutations found on each of 17 alleles derived from nine WT hybridomas, 12 of which contain Sμ deletions. The mutations were distributed as follows: four alleles with 1 bp, two with 2 bp, and one with 3 bp altered. (Right) Sequences derived from six WT hybridomas with 12 alleles revealed two alleles each with a single bp mutation.
Discussion
Implications of ISR in Constructs for the CSR Mechanism.
Intra-S region recombination, as most clearly evidenced by large Sμ deletions, seemed likely to be related to CSR, as it has been observed in cells that undergo or have undergone bona fide CSR and also involves cleavage and joining of S region sequences. We now show that several different transcribed ISR substrates undergo a high rate of ISR after introduction into CSR-competent pro-B cell lines. The 18–8 cell line expresses substantial constitutive levels of AID and undergoes relatively high levels of construct ISR without stimulation. This finding correlates with studies that showed the 18–81 subclone of 18–8 has constitutive AID levels sufficient to induce SM in a reporter substrate (28). In contrast, AID levels are much lower in the A20 subclone but greatly increase after LPS treatment. Thus, by introducing the μ-GFP construct into A20 cells, we could clearly correlate construct ISR with AID induction, although these studies did not rule out induction of other factors that enhance ISR deletion. However, we have confirmed the AID dependence of ISR suggested by assaying for endogenous Sμ ISR events in AID-deficient B cells and also showing that these events are accompanied by mutations. Therefore, we conclude that most ISR is related to normal CSR events.
We have found a very high frequency of ongoing ISR construct deletions by Southern blotting techniques, which detect only large deletions; clearly, many smaller deletions could be present but detectable only by sequence analysis. Likewise, only large deletions could be detected by our endogenous studies. Based on the ISR substrate studies, we suggest that CSR initiation does not require coordinate interaction (e.g., synapsis) between two heterologous S regions. Thus, ISR events appear to be resolved via internal S region recombination after a CSR initiation event in an S region. In this regard, large ISR deletions may result from joining of initiating lesions that occur at some distance within a given S region and not reflect the overall frequency of initiated events directly rejoined or joined to very proximal sequences. The finding of high levels of ISR may also have implications for control of CSR via differential ability of the various S regions to target DNA breaks. In preliminary studies, we failed to detect deletions in downstream S regions (γ1 and γ2b) in the WT IgM-producing hybridomas that had not undergone CSR (D.D.D. and F.W.A., unpublished data). However, when Sμ is removed via targeted deletion, such downstream alterations were observed (22). In this regard, Sμ may be intrinsically more prone to S region cleavage (and ISR) with the downstream acceptor S region break being rate-limiting for efficient CSR. Overall, the ISR substrates may prove valuable for further analyses of the mechanism of CSR, particularly after introduction of AID and ISR substrates into various types of mutant cells.
Potential Roles of AID in CSR as Reflected by ISR.
To date, the exact mechanism by which AID affects CSR, SM, and gene conversion is unknown. We clearly demonstrate that high levels of ISR require AID expression. Likewise, by examining hybridomas, we demonstrated S region mutations within alleles of a single B cell that has undergone ISR and not CSR. Our finding that downstream Sμ mutations occur preferentially in WT hybridomas that have undergone ISR supports the notion ISR occurs via a mechanism related to that of CSR. Previous studies demonstrated mutations at CSR junctions (3) and also within upstream Sμ sequences from WT, but not AID-deficient, activated B cells that had been activated but not yet undergone CSR (14, 15). We note, however, that although the latter activated B cells had not undergone bona fide CSR, it is possible that a significant portion of these cells had undergone the related ISR, and that some detected mutations could have resulted from error-prone ISR repair.
Most available evidence suggests that AID plays a role in initiating CSR (14, 15). However, a postcleavage role for AID in SM was raised by the unexpected finding of WT levels of double-stranded breaks in the variable region genes of activated AID-deficient B cells (30, 31). In the context of a postcleavage role in CSR, AID might function to recruit error-prone factors to resolve S region breaks (6). Thus, S region breaks would be “open and shut” in the absence of AID, with the result being no detectable recombination. In the context of a postcleavage model, our findings may suggest S region breaks in activated AID-deficient cells would generally be resolved via a mechanism that only rarely results in detectable deletions or mutations. In the context of an initiation model, our finding of a low level of apparent ISR in hybridomas derived from AID-deficient cells was unexpected. However, although AID is not expressed in the hybridoma fusion partner or in the hybridomas (data not shown), we cannot formally rule out ISR via transient AID expression from the fusion partner at the time of fusion. Alternatively, our findings may indicate that transcribed Sμ regions are relatively unstable even in the absence of AID expression. In this regard, AID-independent ISR recombination may be mechanistically distinct from AID-dependent ISR and CSR. Yet another possibility is that S regions may be intrinsically prone to DNA breaks/recombination and AID functions to augment the efficiency of this process thereby functioning to augment an initiating mechanistic step (2, 11, 32).
Supplementary Material
Acknowledgments
We thank Fritz Melchers, David Schatz, and Jianzhu Chen for critically reading this manuscript. This work was supported by National Institutes of Health Grant AI31541 (to F.W.A.), a grant from the Charles Hood Foundation and the Lymphoma Research Foundation (to J.P.M.), a fellowship from the National Cancer Institute of Canada with funds provided by Terry Fox Run (to A.A.Z.), and National Institutes of Health Training Grant AI07512 (to M.T.) F.W.A. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- CSR
class switch recombination
- ISR
intra-switch recombination
- AID
activation-induced deaminase
- IgH
Ig heavy chain
- S region
switch region
- SM
somatic mutation
- LPS
lipopolysaccharide
- GFP
green fluorescent protein
- WT
wild type
References
- 1.Bassing C H, Swat W, Alt F W. Cell. 2002;109,Suppl.:S45–S55. doi: 10.1016/s0092-8674(02)00675-x. [DOI] [PubMed] [Google Scholar]
- 2.Manis J P, Tian M, Alt F W. Trends Immunol. 2002;23:31–39. doi: 10.1016/s1471-4906(01)02111-1. [DOI] [PubMed] [Google Scholar]
- 3.Dunnick W, Hertz G Z, Scappino L, Gritzmacher C. Nucleic Acids Res. 1993;21:365–372. doi: 10.1093/nar/21.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee C G, Kondo S, Honjo T. Curr Biol. 1998;8:227–230. doi: 10.1016/s0960-9822(98)70087-9. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs H, Bross L. Curr Opin Immunol. 2001;13:208–218. doi: 10.1016/s0952-7915(00)00206-5. [DOI] [PubMed] [Google Scholar]
- 6.Papavasiliou F N, Schatz D G. Cell. 2002;109,Suppl.:S35–S44. doi: 10.1016/s0092-8674(02)00706-7. [DOI] [PubMed] [Google Scholar]
- 7.Stavnezer J. Curr Top Microbiol Immunol. 2000;245:127–168. doi: 10.1007/978-3-642-59641-4_6. [DOI] [PubMed] [Google Scholar]
- 8.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 9.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, et al. Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 10.Okazaki I, Kinoshita K, Muramatsu M, Yoshikawa K, Honjo T. Nature (London) 2002;416:340–344. doi: 10.1038/nature727. [DOI] [PubMed] [Google Scholar]
- 11.Honjo T, Kinoshita K, Muramatsu M. Annu Rev Immunol. 2002;20:165–196. doi: 10.1146/annurev.immunol.20.090501.112049. [DOI] [PubMed] [Google Scholar]
- 12.Wuerffel R A, Du J, Thompson R J, Kenter A L. J Immunol. 1997;159:4139–4144. [PubMed] [Google Scholar]
- 13.Chen X, Kinoshita K, Honjo T. Proc Natl Acad Sci USA. 2001;98:13860–13865. doi: 10.1073/pnas.241524898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen S, Casellas R, Reina-San-Martin B, Chen H T, Difilippantonio M J, Wilson P C, Hanitsch L, Celeste A, Muramatsu M, Pilch D R, et al. Nature (London) 2001;414:660–665. doi: 10.1038/414660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagaoka H, Muramatsu M, Yamamura N, Kinoshita K, Honjo T. J Exp Med. 2002;195:529–534. doi: 10.1084/jem.20012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paull T T, Rogakou E P, Yamazaki V, Kirchgessner C U, Gellert M, Bonner W M. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 17.Dunnick W, Stavnezer J. Mol Cell Biol. 1990;10:397–400. doi: 10.1128/mcb.10.1.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alt F W, Rosenberg N, Casanova R J, Thomas E, Baltimore D. Nature (London) 1982;296:325–331. doi: 10.1038/296325a0. [DOI] [PubMed] [Google Scholar]
- 19.Burrows P D, Beck-Engeser G B, Wabl M R. Nature (London) 1983;306:243–246. doi: 10.1038/306243a0. [DOI] [PubMed] [Google Scholar]
- 20.Winter E, Krawinkel U, Radbruch A. EMBO J. 1987;6:1663–1671. doi: 10.1002/j.1460-2075.1987.tb02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hummel M, Berry J K, Dunnick W. J Immunol. 1987;138:3539–3548. [PubMed] [Google Scholar]
- 22.Gu H, Zou Y R, Rajewsky K. Cell. 1993;73:1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- 23.Bottaro A, Young F, Chen J, Serwe M, Sablitzky F, Alt F W. Int Immunol. 1998;10:799–806. doi: 10.1093/intimm/10.6.799. [DOI] [PubMed] [Google Scholar]
- 24.Li S C, Rothman P, Boothby M, Ferrier P, Glimcher L, Alt F W. Control of Immunoglobulin Heavy Chain Constant Region Gene Expression. New York: Plenum; 1991. [DOI] [PubMed] [Google Scholar]
- 25.Shaw A C, Swat W, Ferrini R, Davidson L, Alt F W. J Exp Med. 1999;189:123–129. doi: 10.1084/jem.189.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siden E J, Baltimore D, Clark D, Rosenberg N E. Cell. 1979;16:389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- 27.Manis J P, Dudley D, Kaylor L, Alt F W. Immunity. 2002;16:607–617. doi: 10.1016/s1074-7613(02)00306-0. [DOI] [PubMed] [Google Scholar]
- 28.Bachl J, Carlson C, Gray-Schopfer V, Dessing M, Olsson C. J Immunol. 2001;166:5051–5057. doi: 10.4049/jimmunol.166.8.5051. [DOI] [PubMed] [Google Scholar]
- 29.Luby T M, Schrader C E, Stavnezer J, Selsing E. J Exp Med. 2001;193:159–168. doi: 10.1084/jem.193.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bross L, Muramatsu M, Kinoshita K, Honjo T, Jacobs H. J Exp Med. 2002;195:1187–1192. doi: 10.1084/jem.20011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papavasiliou F N, Schatz D G. J Exp Med. 2002;195:1193–1198. doi: 10.1084/jem.20011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian M, Alt F W. J Biol Chem. 2000;275:24163–24172. doi: 10.1074/jbc.M003343200. [DOI] [PubMed] [Google Scholar]
- 33.Chen F, Ma L, Parrini M C, Mao X, Lopez M, Wu C, Marks P W, Davidson L, Kwiatkowski D J, Kirchhausen T, et al. Curr Biol. 2000;10:758–765. doi: 10.1016/s0960-9822(00)00571-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.