Abstract
Background: Evidence for the effectiveness of topical treatments, in providing symptomatic relief from ocular allergy, remains uncertain.
Aims: To assess the effectiveness and relative efficacy of topical treatments for the management of seasonal allergic conjunctivitis.
Design of study: A systematic review and meta-analysis.
Setting: A literature search of the Cochrane Library, Medline, and EMBASE bibliographic databases.
Method: Double-masked randomised controlled trials were identified, that compared the use of topical mast cell stabilisers (sodium cromoglycate, nedocromil, lodoxamide) with placebo, topical antihistamines with placebo, and topical mast cell stabilisers with topical antihistamines.
Results: A meta-analysis of six trials showed that patients using sodium cromoglycate were 17 times (95% confidence interval [CI] = 4 to 78) more likely to perceive benefit compared with those using a placebo, although this estimate may be partially influenced by publication bias. Five trials indicated that those patients using nedocromil were 1.8 times (95% CI = 1.3 to 2.6) more likely to perceive their allergy to be moderately or totally controlled than those using a placebo. Four trials showed that those using antihistamines were 1.3 times (95% CI = 0.8 to 2.2) more likely to perceive a ‘good’ treatment effect than those using mast cell stabilisers, although this beneficial effect was not statistically significant. Limited evidence suggests that antihistamines might have a faster therapeutic effect compared to mast cell stabilisers.
Conclusion: Overall, these findings confirm the benefit of topical mast cell stabilisers and antihistamines over placebo for the treatment of allergic conjunctivitis. There is, however, insufficient evidence to recommend the use of one type of medication over another. Treatment preferences should therefore be based on convenience of use (with reduced frequency of instillation for some preparations), patient preference, and costs, especially as important side effects were not reported with any medication.
Keywords: clinical trials; conjunctivitis; conjunctivitis; allergic; meta-analysis; review, systematic; topical administration
Introduction
FIFTEEN per cent of eye related consultations in general practice are due to allergic conjunctivitis,1 which may or may not be accompanied by rhinitis (so called allergic rhinoconjunctivitis).2 Half of these cases are likely to be diagnosed with seasonal allergic conjunctivitis,3 which is a Type 1, IgE-mediated hypersensitivity to grass or tree pollen. Hence, most are seen when pollens are present in the atmosphere (typically between April and August in the United Kingdom [UK]). Cases may present severely in the rare event of excessive allergen exposure.
Recommended treatments for symptoms of allergic conjunctivitis — namely, ocular itching and redness with tearing, and accompanying nasal congestion — include avoidance of the offending antigen(s), topical mast cell stabilisers, and topical antihistamines (with and without a vasoconstrictor).4 Systemic antihistamines can be used, these usually being reserved for those with atopic symptoms affecting other organ systems. Refractory cases and those with more serious sight-threatening atopic conditions, such as vernal and atopic keratoconjunctivitis, may require treatment with topical steroids, although this is usually administered under specialist supervision.
Most patients presenting with seasonal allergic conjunctivitis are treated using either topical mast cell stabilisers or antihistamines. However, the evidence base for the comparative effectiveness of these topical treatments in providing symptomatic relief from ocular allergy remains uncertain.
We aimed to systematically evaluate the effectiveness of:
topical mast cell stabilisers when compared with placebo,
topical antihistamines when compared with placebo, and
topical mast cell stabilisers compared with topical antihistamines
in providing symptomatic relief from seasonal allergic conjunctivitis.
This is, as far as we are aware, the first systematic review on this subject. The role of systemic antihistamines, topical and systemic corticosteroids, and non-steroidal anti-inflammatory drugs in the treatment of allergic conjunctivitis are the subjects of future reviews.
Method
Study inclusion and search strategy
A systematic review of all double-masked randomised crossover and non-crossover trials was carried out, comparing (a) topical mast cell stabilisers with placebo, (b) topical antihistamines with placebo, and (c) topical mast cell stabilisers with antihistamines, in the management of seasonal allergic conjunctivitis. One reviewer completed the search in August 2001 in accordance with Cochrane guidelines for locating and selecting studies.5 Studies were identified from the Cochrane Eyes and Vision Group trials register, derived from the Cochrane central register of trials on the Cochrane Library (Issue 2, 2001), Medline (1966–2001), and EMBASE (1980–2001) databases. A detailed text word and MeSH heading (for Medline only) search strategy was used (see Supplementary appendix 1). Bibliographies of relevant articles were searched for additional references.
HOW THIS FITS IN
What do we know?
Allergic conjunctivitis affects up to one-fifth of the United Kingdom population, presenting a considerable burden to primary healthcare services. Treatment for this condition often involves either topical mast cell stabilisers or antihistamines. However, the evidence for the relative effectiveness of these topical treatments in providing symptomatic relief from ocular allergy remains uncertain.
What does this paper add?
A systematic review of 40 double-masked randomised controlled trials indicates that treatment of allergic conjunctivitis with either topical mast cell stabilisers or antihistamines confers benefit in alleviating symptoms when compared with a placebo. A meta-analysis did not show any difference in subjective symptoms between those treated with topical antihistamines when compared with those treated with topical mast cell stabilisers.
Selection of trials and assessment of quality
Titles and abstracts of studies identified from the searches were reviewed. Studies were only considered relevant if the abstract stated that a randomised controlled trial of relevant treatments had been carried out. Full-text versions of potentially relevant articles were obtained and assessed for a minimum standard of quality. Only trials that met the inclusion criteria; that is, concealed allocation of treatment, contained subjective assessment of treatment efficacy, and documented patient inclusion criteria, were included in the review. Crossover trials were only included if a suitable wash-out period between treatments was reported. The majority of studies did not detail the method of treatment allocation, and so this could not be used to assess the individual quality of the studies.
Data extraction and statistical methods
Two reviewers extracted data, in accordance with Cochrane guidelines,5 onto a standard pro forma (Microsoft Excel 97 spreadsheet). The extraction process was completed on two separate occasions. The mean difference in subjective symptoms (for ocular itching or general scores of discomfort) and the standard error of the difference between treatment groups were often not reported. The perceived benefits for different treatments (that is, topical mast cell stabilisers, antihistamines, and placebo) were more routinely reported. Hence, the odds ratios and 95% confidence intervals (CI) of perceived benefit for different topical treatments were calculated using a meta-analysis command (METAN) in STATA (STATA 7.0 for Windows). In the absence of any statistically significant between-study heterogeneity, a fixed pooled effect is reported.6 Where statistically significant (P<0.05) between-study heterogeneity was identified, we performed meta-analysis using a random-effects model. Statistical heterogeneity between studies was examined using the χ2 test. Funnel plots were used to assess whether there was evidence of publication bias.7 An Egger test for funnel plot asymmetry and a Begg test for publication bias were also performed.8,9 Quantitative analyses of outcomes were performed on an intention-to-treat basis, where possible.
Results
Titles and abstracts from 140 studies were identified, and full-text versions of 49 potentially relevant articles were obtained and assessed for quality. Forty studies satisfied our pre-specified inclusion criteria (see Supplementary figure 1).
Topical mast cell stabilisers versus placebo
Three different types of topical mast cell stabiliser (sodium cromoglycate, nedocromil, and lodoxamide) were compared with placebo in providing symptomatic relief from allergic conjunctivitis.
Sodium cromoglycate versus placebo. Seventeen double-masked studies that compared use of topical sodium cromoglycate with placebo were identified (a reference list is available from the authors). Eight studies recorded subjective symptoms while using treatment and placebo interventions, including ocular itching, burning, soreness, and lacrimation.10-17 Of these, five studies reported an improvement in a variety of subjective symptoms while using topical sodium cromoglycate preparations,11-13,15,17 whereas the remaining three trials found no difference in symptoms between treatment groups.10,14,16 Formal meta-analysis was not possible because mean scores or measures of accuracy (standard deviation or standard error) were not reported.
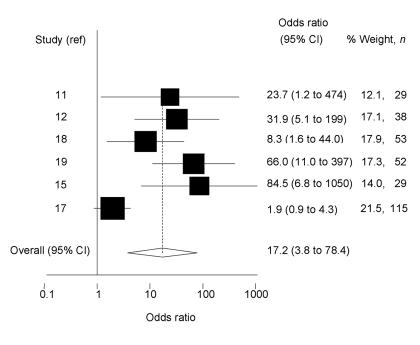
Ascertainment of preferred treatment in crossover trials, and overall assessment of perceived treatment benefit in non-crossover trials was more consistently reported. The characteristics of these six small trials are detailed in Supplementary table 1.11,12,15,17-19 Overall, a random-effects estimate (owing to considerable heterogeneity between estimates; χ2 = 23.2, degrees of freedom [df] = 5, P<0.001) showed that those using topical sodium cromoglycate preparations were 17 times (95% CI = 4 to 78) more likely to perceive benefit than those using placebo (Figure 1). Trials reporting marked and statistically significant benefits of active treatment over placebo were mostly small, raising the possibility of publication bias. The Egger test for publication bias was statistically significant (P = 0.02), although the Begg test was not (P = 0.45).
Figure 1.
Odds ratio and 95% CI of perceived benefit of using sodium cromoglycate compared with placebo. Study reference is indicated on the y-axis (in alphabetical order of author). The pooled estimate, based on a random-effects model, is shown by a dashed vertical line indicating benefit from sodium cromoglycate.
No important side effects were reported with the active treatment, although one historic study that used phenylethanol reported stinging on instillation in both treatment and placebo groups.18
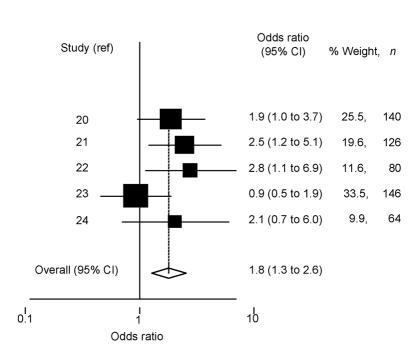
Nedocromil sodium versus placebo. We found five double-masked randomised controlled trials that compared use of topical nedocromil sodium with placebo (over at least 1 month) for the treatment of seasonal allergic conjunctivitis (Supplementary table 2).20-24 To facilitate masking, placebo drops were coloured yellow with riboflavin (0.005%) in all studies, to make them indistinguishable from the active preparation. Subjective symptoms (including itching and overall eye condition) were less pronounced in those using nedocromil sodium compared with those using placebo. The differences were statistically significant in three of these studies,20,22,23 and of borderline significance in the remaining two studies.21,24 Heterogeneity in the approaches to subjective recording of symptoms and presentation of results did not allow for a formal meta-analysis (this was also true of clinician-based assessment of treatment efficacy). Patient-perceived total and moderate effectiveness of treatment was reported in all studies (Supplementary table 2). A fixed-effects estimate (as differences between estimates were not statistically significant; χ2 = 5.15, df = 4, P = 0.27) showed that patients using nedocromil sodium were 1.8 times (95% CI = 1.3 to 2.6) more likely to report that their symptoms were moderately or totally controlled than those using placebo (Figure 2). Tests for publication bias were not statistically significant (Egger test P = 0.55, Begg test P = 0.46). Apart from an unpleasant taste immediately after instillation of the active treatment, no other important side effects were reported.
Figure 2.
Odds ratio and 95% CI of perceived benefit of using nedocromil sodium compared with placebo. Study reference is indicated on the y-axis (in alphabetical order of author). The pooled estimate, based on a fixed-effects model, is shown by a dashed vertical line indicating benefit from nedocromil sodium (2%).
Lodoxamide tromethamine versus placebo. Only one randomised controlled trial of 4 weeks duration compared the use of lodoxamide tromethamine 0.1% with placebo for the treatment of allergic conjunctivitis in adults.25 Those using lodoxamide (n = 14) reported significantly fewer symptoms of lacrimation, burning and itching, photophobia, and eyelid swelling compared with those using placebo (n = 13). Fewer patients treated with lodoxamide (n = 2/14) compared with the placebo group (n = 11/13) complained of symptoms requiring additional pharmacological treatment (P<0.002).25 Three other studies using conjunctival provocation tests to a variety of allergens showed greater short-term symptomatic relief in those using lodoxamide compared with placebo. Cytological assessment in these three provocation studies found fewer inflammatory cells in the tear fluid of the lodoxamide-treated group. Again, owing to heterogeneity in methods and presentation of results, a meta-analysis of these studies could not be performed. No side effects associated with use of the active treatment were reported.
Topical mast cell stabilisers versus placebo. Six studies comparing sodium cromoglycate with placebo (Supplementary table 1), five studies comparing nedocromil sodium with placebo (Supplementary table 2), and one study comparing lodoxamide tromethamine with placebo, were pooled to give a combined estimate of the efficacy of topical mast cell stabilisers over placebo. Overall, a random-effects model (as there was considerable heterogeneity between estimates χ2 = 45.4, df = 11, P<0.001) showed that patients using mast cell stabilisers were 4.9 times (95% CI = 2.5 to 9.6) more likely to perceive benefit than those using placebo. However, caution should be used with this pooled estimate, as the outcomes of benefit between treatments used, although similar, were not the same. Also, there was marked evidence of publication bias (Begg test P = 0.005, Egger test P<0.001) and differences in the effect between types of treatments (P = 0.004 for the difference in benefit between sodium cromoglycate versus placebo, and nedocromil sodium versus placebo). No trials were identified directly comparing the use of one mast cell stabiliser with another.
Topical antihistamines versus placebo
Nine double-masked randomised controlled trials (consisting of crossover and non-crossover designs) were identified: six studies compared treatment of levocabastine with placebo, one study compared azelastine hydrochloride with placebo, one study compared emedastine with placebo, and one further study from the 1970s compared antazoline phosphate with placebo (Supplementary table 3). Because of the rapid mode of action of antihistamines, most studies used short-term conjunctival provocation tests to a variety of allergens, sometimes performed outside the pollen season, to establish the relative efficacy of topical antihistamines and placebo (Supplementary table 3). A variety of symptoms and signs, including itching, redness, burning, and swelling, were graded using scales ranging from 0 (none) to 3 (severe),26-29 or 0 (none) to 6 (severe),30 or using subjective visual analogue scales.31 Formal meta-analysis was not possible, as most studies did not tabulate the mean scores and error associated with these scores. Often P-values associated with the difference between treatment groups were given (summarised in Supplementary table 3), which did not allow the degree of benefit to be gauged. Despite this, most studies showed improvement in symptoms post-provocation, and in allergic conjunctivitis, especially for symptoms of itching (the hallmark symptom of allergic conjunctivitis), in those treated with antihistamines compared with those given placebo. There was no evidence from the randomised controlled trials identified to support the use of one type of topical antihistamine over another.
Topical mast cell stabilisers versus topical antihistamines
Eight double-masked randomised controlled trials comparing the use of topical mast cell stabilisers (five studies evaluated sodium cromoglycate,32-36 one lodoxamide,37 and two nedocromil sodium38,39) with one type of topical antihistamine (levocabastine) were identified. Two were short-term trials comparing the response to conjunctival provocation with a variety of grass pollens, 15 minutes after treatment with nedocromil sodium and levocabastine,38 and after 18 days of treatment with sodium cromoglycate and 4 hours with levocabastine,32 in studies with 24 and 50 participants, respectively. Six trials established the longer-term response to treatment with mast cell stabilisers and levocabastine in studies ranging from 14 days37 to 4 months in duration,33 with 37–110 study participants.34,35 Placebo drops were used to facilitate masking between treatment groups requiring different daily dosage (for example, sodium cromoglycate four times a day, levocabastine twice a day), ensuring an equivalent daily instillation of drops. No trials were found comparing topical mast cell stabilisers with antihistamine and vasoconstrictor preparations.
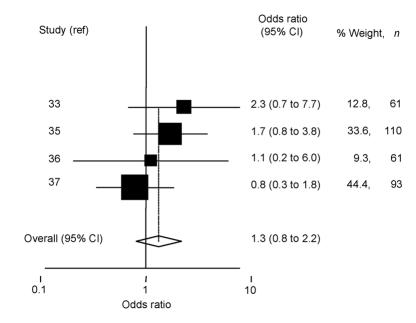
A variety of subjective symptom scores, such as itching, tearing, and burning, as well as signs, such as redness, were graded using scales from 0 (none) to 3 (severe)32,33,35,37,38 or visual analogue scales.34,36 These were used as separate scores, or summed to give overall symptom scores. As with earlier comparisons, formal meta-analysis was not possible, as most studies did not tabulate the mean scores and error associated with these measures. Despite this, differences in scores between treatment groups were reported as not being statistically significant in the six longer-term studies. A statistically significant reduction in itching and redness (P<0.05) in those treated with antihistamines was reported in the two short-term provocation studies.32,38 Mean scores were tabulated in one of these studies, allowing the comparative benefit of topical antihistamine over sodium cromoglycate to be gauged.32 The benefit of antihistamine use in short-term studies was confirmed in interim results (at 2 weeks) from one of the longer studies (4 weeks).35 Patient-perceived ‘excellent’ or ‘good’ treatment efficacy was reported in four of the six longer-term studies (summarised in Supplementary table 4). A fixed-effect estimate (as there was little heterogeneity between estimates; χ2 = 2.72, df = 3, P = 0.44) showed that those using levocabastine were 1.3 times (95% CI = 0.8 to 2.2) more likely to perceive a ‘good’ treatment effect than those using mast cell stabilisers (Figure 3). However, as indicated by the 95% CIs, this difference was not statistically significant. Removal of the one study that compared nedocromil sodium with levocabastine (instead of sodium cromoglycate) slightly increased the perceived benefit of levocabastine over sodium cromoglycate (odds ratio = 1.7, 95% CI = 0.9 to 3.2), but this again was not statistically significant. There was no evidence of publication bias for either of these estimates (Begg tests P = 1.00, 1.00; Egger tests P = 0.84, 0.77, respectively).
Figure 3.
Odds ratio and 95% CI of perceived good or excellent treatment efficacy with topical mast cell stabilisers versus antihistimines. Study reference is indicated on the y-axis (in alphabetical order of author). The pooled estimate, based on a fixed-effects model, is shown by a dashed vertical line indicating benefit from topical antihistamine.
The use of concomitant medications (such as systemic antihistamines, ocular and nasal medications) among treatment groups as a rescue medication in cases of severe symptoms was not routinely reported and hence could not be analysed further. Despite concerns about the sedative effect with systemic use of antihistamines, there were no side effects associated with topical use.
Discussion
Our systematic review of double-masked, randomised controlled trials confirms the benefit of topical sodium cromoglycate and antihistamines over placebo preparations for the treatment of seasonal allergic conjunctivitis. There is insufficient evidence to support the use of one class of active medication over another. We found limited evidence to suggest that topical antihistamines have a faster mode of action than mast cell stabilisers (especially sodium cromoglycate), however, there was little difference in treatment efficacy after 2 weeks.
Topical mast cell stabilisers
Meta-analysis revealed that those using topical sodium cromoglycate were 17 times (95% CI = 4 to 78) more likely to perceive benefit than those using placebo. However, the majority of trials identified were small, there was a large amount of heterogeneity between study estimates, and the pooled estimate had wide confidence intervals. Heterogeneity between estimates may be explained by differences in sample ages, timing of the study and/or active preparations used (see Supplementary table 1). The combined estimate may therefore partially overestimate the beneficial effect of sodium cromoglycate, as some studies that reported no differences in subjective symptoms between treatment groups did not have sufficient data for inclusion (a reference list is available from the authors). In addition, the largest study found less difference in preference between treatment groups and an Egger test for publication bias was statistically significant (although a Begg test was not).17 Hence, the role of publication bias, where small studies showing beneficial effects of the active treatment are published in preference to those that show little difference, cannot be excluded. Evidence of publication bias was also found in a recent systematic review comparing the use of sodium cromoglycate with placebo in the treatment of asthma in childhood,40 consistent with the findings of this review.
The patient-perceived benefit of nedocromil sodium compared with placebo appears less pronounced (1.8 times, 95% CI = 1.3 to 2.6) than the benefit of sodium cromoglycate over placebo. However, the estimate associated with nedocromil sodium may be more reliable as it is derived from studies with more participants (compare Supplementary table 1 with Supplementary table 2), is consistent between studies (with no apparent publication bias), and associated with narrower confidence intervals. Hence, although these studies consistently report improvement in symptoms of allergic conjunctivitis in those using different topical mast cell stabilisers versus placebo, there is no clear evidence to support the use of one type of mast cell stabiliser over another. Surprisingly, no trials were found comparing the use of one type of mast cell stabiliser with another. Treatment preferences should therefore be based on convenience of use (with reduced frequency of instillation for nedocromil preparations), cost considerations, and patient and professional preferences. Overall, patients using topical mast cell stabilisers (sodium cromoglycate, nedocromil sodium, or lodoxamide tromethamine) were more likely to perceive benefit than those using placebo (4.9 times, 95% CI = 2.5 to 9.6). However, there was considerable variability between estimates and evidence of publication bias. Further data from large-scale unpublished studies and published studies that have not presented data on the benefit of treatment over placebo are needed to explore the potential influence of publication and/or reporting bias.
Topical antihistamines
Topical antihistamines appear to be associated with fewer allergic symptoms compared with the use of placebo. Clinical consensus,4 experimental,26 and laboratory studies41 have all shown rapid modes of action of antihistamines, especially in comparison with mast cell stabilisers. This systematic review also provides limited evidence that topical antihistamines have a quicker therapeutic effect compared with mast cell stabilisers in protecting against allergic symptoms. Conjunctival provocation studies are often used to establish the short-term efficacy of antihistamine preparations. However, the relevance of these acute studies to chronic environmental allergen exposure in the pollen season is uncertain and has yet to be clarified. Evidence from long-term studies, examining environmental exposure to allergens, showed that patient-perceived treatment efficacy among those using topical antihistamines was slightly better than those using mast cell stabiliser preparations (1.3 times better, 95% CI = 0.8 to 2.2). This effect was slightly stronger (1.7 times, 95% CI = 0.9 to 3.2) when one type of mast cell stabiliser (sodium cromoglycate) was compared with topical antihistamines. However, neither of these differences were statistically significant, indicating that there is no real difference in perceived benefit between types of medication, or that there were insufficient studies for a beneficial effect to be found.
Implications for care
Our systematic review was based on all trials identified by a systematic search of the literature and three major electronic databases/registers. A meta-analysis of double-masked, random controlled trials supports the role of topical mast cell stabilisers and antihistamines over placebo for the treatment of allergic conjunctivitis. There is, however, insufficient evidence to recommend the use of one type of topical medication over another, and treatment preferences should be based on convenience of use (with reduced frequency of instillation for some preparations), patient preference, and costs,42 especially as no important side effects were reported with any medication. Larger trials, standardised in method and presentation of results, are needed to distinguish between different topical treatments, before the increased expense of newer topical preparations can be fully justified. Limited evidence may indicate that topical antihistamines have a quicker mode of action, and hence may be justified if a more rapid therapeutic effect is warranted, however further trial evidence is needed. It is noteworthy that prolonged use of medications that contain both antihistamine (antazoline) and a vasoconstrictor (naphazoline) should be avoided as this can cause reactive hyperaemia.43
Supplementary information
Additional information accompanies this paper at: http://www.rcgp.org.uk/journal/index.asp
Supplementary Material
Acknowledgments
This paper is partly based on a chapter written by the authors in: Wormald R, Smeeth L, Henshaw K (eds). Evidence Based Ophthalmology, available from BMJ Books (London, UK).
Supplementary appendix 1. Search strategy.
The following strategy was used to search CENTRAL on The Cochrane Library:
#1 CONJUNCTIVITIS-ALLERGIC*:ME
#2 CONJUNCTIVIT*
#3 (#1 or #2)
#4 MAST-CELLS*:ME
#5 CROMOLYN-SODIUM*:ME
#6 (MAST near CELL*)
#7 MAST-CELL*
#8 ((CHROMO* or CROMO*) near SODIUM)
#9 ((CHROMO* or CROMO*) near DISODIUM)
#10 ((NEDOCHROM* or NEDOCROM*) near SODIUM)
#11 ((NEDOCHROM* or NEDOCROM*) near DISODIUM)
#12 LODOXAMIDE or OLOPATADINE
#13 (((((((((#4 or #5) or #6) or #7) or #8) or #9) or #10) or #11) or #12)
#14 HISTAMINE-H1-ANTAGONISTS:ME
#15 ANTAZOLINE:ME
#16 CHLORPHENIRAMINE:ME
#17 CYCLIZINE:ME
#18 TERFENADINE*:ME
#19 (ANTIHISTAMIN* or ANTI-HISTAMIN*)
#20 (((AZELASTIN* or LEVOCABASTIN*) or EMEDASTIN*) or CHLORPHEN*)
#21 (((ACRIVASTIN* or ATIRIZIN*) or DESLORATADIN*) or FEXOFENADIN*)
#22 (((LEVOCITIRIZIN* or LORATIDIN*) or MIZOLASTIN*) or TERFEN*)
#23 ((((((((#14 or #15) or #16) or #17) or #18) or #19) or #20) or #21) or #22)
#24 (#13 or #23)
#25 (#3 and #24)
The following strategy was used to search MEDLINE on SilverPlatter:
#1 “CONJUNCTIVITIS,-ALLERGIC”/ all subheadings
#2 “CONJUNCTIVITIS”/ all subheadings
#3 #2 and (PY=1966-1985)
#4 (ALLERGIC or SEASONAL or PERENNIAL or HAY?FEVER or ENVIRONMENTAL or NON?INFECTIOUS or HISTAMINE?INDUC* or CHRONIC or CHEMICAL or ACUTE) in TI,AB
#6 #4 near3 CONJUNCTIV*
#7 #1 or #3 or #6
#8 “MAST-CELLS”/ all subheadings
#9 “CROMOLYN-SODIUM”/ all subheadings
#10 “CHROMONES”/ all subheadings
#11 #10 and (PY=1966-1970)
#12 “MAST CELL” or “MAST CELLS” or MAST?CELL*
#13 (C?ROMO* or NEDOC?ROM*) near (SODIUM or DISODIUM)
#14 LODOXAMIDE or OLOPATADINE
#15 #8 or #9 or #11 or #12 or #13 or #14
#16 “HISTAMINE-H1-ANTAGONISTS”/ all subheadings
#17 “ANTAZOLINE”/ all subheadings
#18 “CHLORPHENIRAMINE”/ all subheadings
#19 “CYCLIZINE”/ all subheadings
#20 “TERFENADINE”/ all subheadings
#21 ANTI?HISTAMIN* or AZELASTIN* or LEVOCABASTIN* or EMEDASTIN* or CHLORPHENAMIN* or ACRIVASTIN* or ATIRIZIN* or DESLORATADIN* or FEXOFENADIN* or LEVOCITIRIZIN* or LORATIDIN* or MIZOLASTIN* or TERFEN?D?IN*
#22 #16 or #17 or #18 or #19 or #20 or #21
#24 #15 or #22
#25 #7 and #23
To identify randomised controlled trials, this search was combined with the Cochrane highly sensitive search strategy phases one and two as contained in The Cochrane reviewer's handbook.
The following strategy was used to search EMBASE on SilverPlatter:
#1 “ALLERGIC-CONJUNCTIVITIS”/ all subheadings
#2 (ALLERGIC or SEASONAL or PERENNIAL or HAY?FEVER or ENVIRONMENTAL or NON?INFECTIOUS or HISTAMINE?INDUC* or CHRONIC or CHEMICAL or ACUTE) in TI,AB
#3 #2 near3 CONJUNCTIV*
#4 #1 or #3
#5 explode “MAST-CELL”/ all subheadings
#6 “CROMOGLYCATE-DISODIUM”/ all subheadings
#7 “LODOXAMIDE”/ all subheadings
#8 “OLOPATADINE”/ all subheadings
#9 “MAST CELL” or “MAST CELLS” or MAST?CELL*
#10 (C?ROMO* or NEDOCROM*) near (DISODIUM or SODIUM)
#11 LODOXAMIDE or OLOPATADINE
#12 #5 or #6 or #7 or #8 or #9 or #10 or #11
#13 explode “HISTAMINE-H1-RECEPTOR-ANTAGONIST”/ all subheadings
#14 ANTI?HISTAMIN* or AZELASTIN* or LEVOCABASTIN* or EMEDASTIN* or CHLORPHENAMIN* or ACRIVASTIN* or ATIRIZIN* or DESLORATADIN* or FEXOFENADIN* or LEVOCITIRIZIN* or LORATIDIN* or MIZOLASTIN* or TERFEN?D?IN*
#15 #13 or #14
#16 #12 or #15
#17 #4 and #16
To identify randomised controlled trials, this search was combined with the following search:
#1 “RANDOMIZED-CONTROLLED-TRIAL”/ all subheadings
#2 “RANDOMIZATION”/ all subheadings
#3 “CONTROLLED-STUDY”/ all subheadings
#4 “MULTICENTER-STUDY”/ all subheadings
#5 “PHASE-3-CLINICAL-TRIAL”/ all subheadings
#6 “PHASE-4-CLINICAL-TRIAL”/ all subheadings
#7 “DOUBLE-BLIND-PROCEDURE”/ all subheadings
#8 “SINGLE-BLIND-PROCEDURE”/ all subheadings
#9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
#10 RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER* in TI,AB
#11 (SINGL* or DOUBL* or TREBL* or TRIPL*) near (BLIND* or MASK*) in TI,AB
#12 #9 or #10 or #11
#13 HUMAN in DER
#14 (ANIMAL or NONHUMAN) in DER
#15 #13 and #14
#16 #14 not #15
#17 #12 not #16
References
- 1.Manners T. Managing eye conditions in general practice. BMJ. 1997;315:816–817. [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson SG, Hourihane JO, Bousquet J, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813–824. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- 3.Freissler KA, Lang GE, Lang GK. Allergic diseases of the lids, conjunctiva, and cornea. Curr Opin Ophthalmol. 1997;8:25–30. doi: 10.1097/00055735-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Leibowitz HM. The red eye. N Engl J Med. 2000;343:345–351. doi: 10.1056/NEJM200008033430507. [DOI] [PubMed] [Google Scholar]
- 5.Clarke M, Oxman AD Cochrane Collaboration. Cochrane Library. Issue 4. Oxford: Update Software; 2001. Cochrane Reviewers' Handbook 4.1.4 [updated October 2001] [Google Scholar]
- 6.Thompson SG. Why sources of heterogeneity in meta-analysis should be investigated. BMJ. 1994;309:1351–1355. doi: 10.1136/bmj.309.6965.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Light RJ, Pillemar DB. Summing up: the science of reviewing research. Cambridge, MA, USA: Harvard University Press; 1984. [Google Scholar]
- 8.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 10.Friday GA, Biglan AW, Hiles DA, et al. Treatment of ragweed allergic conjunctivitis with cromolyn sodium 4% ophthalmic solution. Am J Ophthalmol. 1983;95:169–174. doi: 10.1016/0002-9394(83)90010-7. [DOI] [PubMed] [Google Scholar]
- 11.Greenbaum J, Cockcroft D, Hargreave FE, Dolovich J. Sodium cromoglycate in ragweed-allergic conjunctivitis. J Allergy Clin Immunol. 1977;59:437–439. doi: 10.1016/0091-6749(77)90006-9. [DOI] [PubMed] [Google Scholar]
- 12.Hechanova MG. A double-blind study comparing sodium cromoglycate eye ointment with placebo in the treatment of chronic allergic conjunctivitis. Clinical Trials Journal. 1984;21:59–66. [Google Scholar]
- 13.Leino M, Ennevaara K, Latvala AL, et al. Double-blind group comparative study of 2% nedocromil sodium eye drops with 2% sodium cromoglycate and placebo eye drops in the treatment of seasonal allergic conjunctivitis. Clin Exp Allergy. 1992;22:929–932. doi: 10.1111/j.1365-2222.1992.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 14.Montan P, Zetterstrom O, Eliasson E, Stromquist LH. Topical sodium cromoglycate (Opticrom) relieves ongoing symptoms of allergic conjunctivitis within 2 minutes. Allergy. 1994;49:637–640. doi: 10.1111/j.1398-9995.1994.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 15.Ruggieri ML, Scorcia G. Double-blind group comparative trial of sodium cromoglycate eye ointment and placebo in the treatment of allergic eye diseases. Ann Allergy. 1987;58:109–112. [PubMed] [Google Scholar]
- 16.Simon-Licht IF, Dieges PH. A double-blind clinical trial with cromoglycate eye drops in patients with atopic conjunctivitis. Ann Allergy. 1982;49:220–224. [PubMed] [Google Scholar]
- 17.van Bijsterveld OP. A double-blind crossover study comparing sodium cromoglycate eye drops with placebo in the treatment of chronic conjunctivitis. Acta Ophthalmol (Copenh) 1984;62:479–484. doi: 10.1111/j.1755-3768.1984.tb08428.x. [DOI] [PubMed] [Google Scholar]
- 18.Lindsay-Miller AC. Group comparative trial of 2% sodium cromoglycate (Opticrom) with placebo in the treatment of seasonal allergic conjunctivitis. Clin Allergy. 1979;9:271–275. doi: 10.1111/j.1365-2222.1979.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 19.Nizami RM. Treatment of ragweed allergic conjunctivitis with 2% cromolyn solution in unit doses. Ann Allergy. 1981;47:5–7. [PubMed] [Google Scholar]
- 20.Blumenthal M, Casale T, Dockhorn R, et al. Efficacy and safety of nedocromil sodium ophthalmic solution in the treatment of seasonal allergic conjunctivitis. Am J Ophthalmol. 1992;113:56–63. doi: 10.1016/s0002-9394(14)75754-x. [DOI] [PubMed] [Google Scholar]
- 21.Leino M, Carlson C, Jaanio E, et al. Double-blind group comparative study of 2% nedocromil sodium eye drops with placebo eye drops in the treatment of seasonal allergic conjunctivitis. Ann Allergy. 1990;64:398–402. [PubMed] [Google Scholar]
- 22.Melamed J, Schwartz RH, Hirsch SR, Cohen SH. Evaluation of nedocromil sodium 2% ophthalmic solution for the treatment of seasonal allergic conjunctivitis. Ann Allergy. 1994;73:57–66. [PubMed] [Google Scholar]
- 23.Moller C, Berg IM, Berg T, et al. Nedocromil sodium 2% eye drops for twice-daily treatment of seasonal allergic conjunctivitis: a Swedish multicentre placebo-controlled study in children allergic to birch pollen. Clin Exp Allergy. 1994;24:884–887. doi: 10.1111/j.1365-2222.1994.tb01811.x. [DOI] [PubMed] [Google Scholar]
- 24.Stockwell A, Easty DL. Group comparative trial of 2% nedocromil sodium with placebo in the treatment of seasonal allergic conjunctivitis. Eur J Ophthalmol. 1994;4:19–23. doi: 10.1177/112067219400400104. [DOI] [PubMed] [Google Scholar]
- 25.Cerqueti PM, Ricca V, Tosca MA, et al. Lodoxamide treatment of allergic conjunctivitis. Int Arch Allergy Immunol. 1994;105:185–189. doi: 10.1159/000236823. [DOI] [PubMed] [Google Scholar]
- 26.Stokes TC, Feinberg G. Rapid onset of action of levocabastine eye-drops in histamine-induced conjunctivitis. Clin Exp Allergy. 1993;23:791–794. doi: 10.1111/j.1365-2222.1993.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 27.Buscaglia S, Paolieri F, Catrullo A, et al. Topical ocular levocabastine reduces ICAM-1 expression on epithelial cells both in vivo and in vitro. Clin Exp Allergy. 1996;26:1188–1196. [PubMed] [Google Scholar]
- 28.Pipkorn U, Bende M, Hedner J, Hedner T. A double-blind evaluation of topical levocabastine, a new specific H1 antagonist in patients with allergic conjunctivitis. Allergy. 1985;40:491–496. doi: 10.1111/j.1398-9995.1985.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 29.Miller J, Wolf EH. Antazoline phosphate and naphazoline hydrochloride, singly and in combination for the treatment of allergic conjunctivitis — a controlled, double-blind clinical trial. Ann Allergy. 1975;35:81–86. [PubMed] [Google Scholar]
- 30.Donshik PC, Pearlman D, Pinnas J, et al. Efficacy and safety of ketorolac tromethamine 0.5% and levocabastine 0.05%: a multicentre comparison in patients with seasonal allergic conjunctivitis. Adv Ther. 2000;17:94–102. doi: 10.1007/BF02854842. [DOI] [PubMed] [Google Scholar]
- 31.Horak F, Berger U, Menapace R, Schuster N. Quantification of conjunctival vascular reaction by digital imaging. J Allergy Clin Immunol. 1996;98:495–500. doi: 10.1016/s0091-6749(96)70081-7. [DOI] [PubMed] [Google Scholar]
- 32.Abelson MB, George MA, Smith LM. Evaluation of 0.05% levocabastine versus 4% sodium cromolyn in the allergen challenge model. Ophthalmology. 1995;102:310–316. doi: 10.1016/s0161-6420(95)31023-8. [DOI] [PubMed] [Google Scholar]
- 33.Frostad AB, Olsen AK. A comparison of topical levocabastine and sodium cromoglycate in the treatment of pollen-provoked allergic conjunctivitis. Clin Exp Allergy. 1993;23:406–409. doi: 10.1111/j.1365-2222.1993.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 34.Odelram H, Bjorksten B, af Klercker T, et al. Topical levocabastine versus sodium cromoglycate in allergic conjunctivitis. Allergy. 1989;44:432–436. doi: 10.1111/j.1398-9995.1989.tb04175.x. [DOI] [PubMed] [Google Scholar]
- 35.Vermeulen J, Mercer M. Comparison of the efficacy and tolerability of topical levocabastine and sodium cromoglycate in the treatment of seasonal allergic rhinoconjunctivitis in children. Pediatr Allergy Immunol. 1994;5:209–213. doi: 10.1111/j.1399-3038.1994.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 36.Wihl JA, Rudblad S, Kjellen H, Blychert LA. Levocabastine eye drops versus sodium cromoglycate in seasonal allergic conjunctivitis. Clin Exp Allergy. 1991;21(Suppl 2):37–38. doi: 10.1111/j.1365-2222.1991.tb01756.x. [DOI] [PubMed] [Google Scholar]
- 37.Richard C, Trinquand C, Bloch-Michel E. Comparison of topical 0.05% levocabastine and 0.1% lodoxamide in patients with allergic conjunctivitis. Study Group. Eur J Ophthalmol. 1998;8:207–216. doi: 10.1177/112067219800800402. [DOI] [PubMed] [Google Scholar]
- 38.Hammann C, Kammerer R, Gerber M, Spertini F. Comparison of effects of topical levocabastine and nedocromil sodium on the early response in a conjunctival provocation test with allergen. J Allergy Clin Immunol. 1996;98:1045–1050. doi: 10.1016/s0091-6749(96)80189-8. [DOI] [PubMed] [Google Scholar]
- 39.Kremer B, Tundermann A, Goldschmidt O. Onset of action, effectiveness and tolerance of levocabastine and nedocromil in topical therapy of seasonal allergic rhinoconjunctivitis. The Deutsche Rhinitis-Studiengruppe. Arzneimittelforschung. 1998;48:924–930. [PubMed] [Google Scholar]
- 40.van der Wouden JC, Tasche MJ, Bernsen RM, et al. Cochrane Collaboration. Cochrane Library. Issue 1. Oxford: Update Software; 2004. Inhaled sodium cromoglycate for asthma in children. [Google Scholar]
- 41.Dechant KL, Goa KL. Levocabastine. A review of its pharmacological properties and therapeutic potential as a topical antihistamine in allergic rhinitis and conjunctivitis. Drugs. 1991;41:202–224. doi: 10.2165/00003495-199141020-00006. [DOI] [PubMed] [Google Scholar]
- 42.Department of Health, Statistics Division. Prescription cost analysis: England 2002. London: Department of Health; 2002. http://www.publications.doh.gov.uk/stats/pca2002.htm (accessed 19 Apr 2004) [Google Scholar]
- 43.Soparkar CN, Wilhelmus KR, Koch DD, et al. Acute and chronic conjunctivitis due to over-the-counter ophthalmic decongestants. Arch Ophthalmol. 1997;115:34–38. doi: 10.1001/archopht.1997.01100150036004. [DOI] [PubMed] [Google Scholar]
- 44.Abelson MB, George MA, Schaefer K, Smith LM. Evaluation of the new ophthalmic antihistamine, 0.05% levocabastine, in the clinical allergen challenge model of allergic conjunctivitis. J Allergy Clin Immunol. 1994;94:458–464. doi: 10.1016/0091-6749(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 45.Zuber P, Pecoud A. Effect of levocabastine, a new H1 antagonist, in a conjunctival provocation test with allergens. J Allergy Clin Immunol. 1988;82:590–594. doi: 10.1016/0091-6749(88)90969-4. [DOI] [PubMed] [Google Scholar]
- 46.Discepola M, Deschenes J, Abelson M. Comparison of the topical ocular antiallergic efficacy of emedastine 0.05% ophthalmic solution to ketorolac 0.5% ophthalmic solution in a clinical model of allergic conjunctivitis. Acta Ophthalmol Scand Suppl. 1999;(228):43–46. doi: 10.1111/j.1600-0420.1999.tb01173.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.