Abstract
Two striking differences between humans and our closest living relatives, chimpanzees and gorillas, are the size of our brains (larger by a factor of three or four) and our life span (longer by a factor of about two). Our thesis is that these two distinctive features of humans are products of coevolutionary selection. The large human brain is an investment with initial costs and later rewards, which coevolved with increased energy allocations to survival. Not only does this theory help explain life history variation among primates and its extreme evolution in humans; it also provides new insight into the evolution of longevity in other biological systems. We introduce and apply a general formal demographic model for constrained growth and evolutionary tradeoffs in the presence of life-cycle transfers between age groups in a population.
We present a theory of life history evolution, which integrates biological theory and the economic theory of capital. We first review the evidence on the evolution of brain size and longevity and present our theory informally. The formal model is outlined next, including its key implication that a more challenging environment, one in which learning by doing plays a larger role, selects for both a larger brain and lower mortality. Three lines of relevant empirical evidence are then considered, and the paper concludes with a brief discussion of the implications of our theory.
The Evolution of Brains and Longevity
The observation that brain size and longevity are associated can be traced to the turn of the 20th century (1). Sacher was the first to rigorously establish the effect on life span of brain size, while controlling for body size, among mammals (2) and then, more specifically, among primates (3). Although spurious correlations here could result from measurement error in body weight (4), subsequent replications (5) have established the robustness of this finding for primates.
To understand this relationship, it is necessary to model the simultaneous action of natural selection on brain size and longevity. However, most studies of brain evolution have ignored longevity and have focused either on the benefits or the costs of brains, but not both. For example, the liveliest current debate concerns whether the benefit of a large brain is to solve ecological or social problems (6). On the cost side, another debate concerns, for example, whether larger brains require smaller guts or lower metabolic rates (7, 8).
The few studies examining the relationship between the brain and longevity also fail to model simultaneous selection; they focus either on the direct impacts of the brain on life span or on the benefits of a longer life span. For example, Sacher (3) offers two proposals: (i) brains directly increase life span by ensuring more precise homeostasis of bodily functions; and (ii) brains delay maturation and lower the reproductive rate, therefore requiring an extension of the life span. Other hypotheses are: (i) larger brains are beneficial to longer-lived animals because they are likelier to experience food shortages when knowledge of the habitat would facilitate survival (9); (ii) larger brains decrease ecological vulnerability to environmental risks and select for increased longevity (10); and (iii) larger brains help maintain tissue differentiation and slow the process of decay leading to senescence (11). Our general theory will analyze how natural selection acts simultaneously on brains and longevity, modeling both costs and benefits. It treats the brain as a costly investment that yields fitness returns in the future, so that these returns also depend on the probability of survival.
The Argument
Our approach builds on life history theory (LHT) in biology. LHT considers the timing of life events and has a particular focus on the age schedules of fertility and mortality. A fundamental tradeoff here is between current and future reproduction. Growth, for example, increases an organism's future energy capture rates and hence future fertility. For this reason, organisms typically have a juvenile phase in which fertility is zero until they reach a size at which reproduction increases fitness more than does growth. Natural selection should optimize the allocation of energy to current reproduction and to future reproduction (via investments in growth and maintenance) so that genetic descendants are maximized (12).
To model the coevolution of brain size and mortality, we generalize existing LHT by treating growth and development as investment in somatic or embodied capital. In a physical sense, embodied capital is organized somatic tissue—muscles, brains, etc. In a functional sense, embodied capital includes strength, skill, knowledge, and other abilities. Because such stocks tend to depreciate with time, allocations to maintenance can also be seen as investments in embodied capital. Thus, the present–future reproductive tradeoff can be understood in terms of optimal investments in embodied capital versus reproduction.
The brain is a special form of embodied capital. On the one hand, neural tissue monitors the organism's internal and external environment and induces physiological and behavioral responses to stimuli (13). On the other hand, the brain has the capacity to transform present experiences into future performance. This is particularly true of the cerebral cortex, which specializes in the storage, retrieval, and processing of experiences. The expansion of the cerebral cortex among higher primates represents an increased investment in this capacity (14, 15).
The action of natural selection on the neural tissue involved in learning, memory, and the processing of stored information depends on the costs and benefits realized over the organism's lifetime. There are potentially substantial energetic costs of growing the brain early in life and of maintaining neural tissue throughout life. Among humans, for example, about 65% of all resting energetic expenditure is used to support the maintenance and growth of the brain in the first year of life (16). Another potential cost of the brain is lower performance early in life. The ability to learn may entail reductions in “preprogrammed” behavioral routines and so decrease early performance. The incompetence of human infants, and even children, in many motor tasks is an example.
Taking these costs into account, the net benefits from the brain tissue involved in learning are then realized over time. In a niche where there is little to learn, a large brain might have a relatively small impact on productivity late in life but higher costs early in life. Natural selection may then tend to favor the small brain. In a more challenging niche, however, although a small brain might be slightly better early in life, partly because of its lower cost, it would be much worse later, and the large brain might be favored instead.
Our theory is that brain size and longevity coevolve for the following reasons. Because the returns to a large brain lie in the future, ecological conditions favoring large brains also favor greater expenditure on survival. Conversely, ecological conditions that lower exogenous mortality favor increased expenditure on survival and hence also much greater investment in brain capital.
In particular, we propose that the coevolution of intelligence and longevity within the nonhominid primates is due to two reinforcing environmental factors. The first of these factors is that the arboreal environment protected against predators. Such lowered mortality favored greater investments in longevity (17) and higher levels of neural capital. The second factor is the phenological complexity of tropical forests with respect to species diversity and fruiting patterns. This factor increased investment in the storage and processing of information (18) and also favored endogenous mortality reduction. Three progressive grade shifts resulted from this coevolutionary process. The first shift toward greater longevity occurred with the evolution of prosimians, probably in response to decreased hazards of mortality (17). Building on this first shift, a second major grade shift toward increased encephalization (brain size, relative to body size), and longevity occurred with the anthropoids (the lineage containing monkeys, apes, and humans), about 35 million years ago. The major defining characteristic of the anthropoids is the reorganization of the sensory system from one dominated by olfaction and hearing to one dominated by binocular color vision. This change is associated with a switch from insect to plant food, involving a manipulating hand and improved hand-eye coordination (15). The third major grade shift in primates toward both increased encephalization and longevity occurs with the hominoid apes. The great apes have the largest brains among nonhominid primates, controlling for body size, and also live more than twice as long as most monkeys. This third shift is most likely due to an increased emphasis on complex extractive foraging techniques and eating ripe fruit (19, 20). Comparison of intercepts among prosimians, monkeys, and apes in regressions of brain size on body size confirms the existence of these three grade shifts (9).
We propose the fourth shift occurred with the evolution of the genus Homo, because of the cognitively challenging hunting and gathering niche created by the formation of the savanna during the late Pliocene–early Pleistocene. Because this niche raised the productivity of the brain, the investment in this organ rose, inducing greater expenditure on mortality reduction, so that longevity rose along with encephalization. The formal model below demonstrates this effect theoretically.
This simultaneous exaggeration of intelligence and longevity within hominids followed entry into a niche that demanded an extended childhood learning phase, where expenditure on this phase was made worthwhile by higher adult productivity. Such a tradeoff requires the possibility of large intergenerational resource flows, like those actually observed. Perhaps an economy permitting such intergenerational trade was an elaboration of the food sharing originally necessitated by large valuable food packages, particularly from hunting.
A Formal Coevolutionary Model of Brain Size and Longevity
The Setup.
Suppose that each individual invests in a brain of size K at time t = 0, with energy cost C(K). The cost of an extra unit of capital, the “marginal cost of capital,” increases as the stock of capital increases. Formally, C: [0, ∞) → [0, ∞) is continuously twice differentiable, with C(K) > 0, C′(0) = 0, and C"(K) > 0, for all K ≥ 0.
This somatic capital stock then creates energy output, given by a continuously twice-differentiable production function F: [0, ∞) × {1, … , T} × A → [0, ∞), where A is a compact interval. For t = 1, … , T, and all α ∈ A, this production function satisfies F(0, t, α) = 0, FK(K, t, α) > 0, FKK(K, t, α) < 0, for all K ≥ 0, and FK(K, t, α) → 0 as K → ∞. That is, an extra unit of capital always yields additional output, but this “marginal product of capital” decreases to zero as the level of capital rises.
The introduction of time, t, into the production function captures important features of the situation. First, there are other relevant somatic stocks—overall body size, for example—and investment in such stocks may continue after neural investment is complete. However, if the time paths of these stocks are exogenous, output net of these investments would tend to initially increase over time. Second, even with a fixed capital stock, K, production will rise at first because of the acquisition of relevant specific skills, that is, “on-the-job training.” A large brain facilitates such learning, but the benefits are fully realized only well into the future. For both of these reasons, there is then taken to be an initial range of ages over which F(K, t, α) is a strictly increasing function of t for all K > 0 and α ∈ A. Third, all somatic stocks, including the brain, seem to become less effective in old age. This effect is captured in a final range of ages over which F(K, t, α) is a strictly decreasing function of t, for all K > 0 and α ∈ A, with a first date (T + 1, say), such that F(K, T + 1, α) ≤ 0.
The effect of the parameter α ∈ A is formulated to reflect a more challenging learning-intensive environment. First, it is assumed that higher values of α lead to lower initial productivity but higher final productivity. That is, if K > 0, Fα(K, t, α) < 0, for all t < t̄, but Fα(K, t, α) > 0, for all t ≥ t̄, for some 1 < t̄ ≤ T.d An increase in α thus captures the steeper rise in productivity associated with learning becoming more significant. Second, FKα(K, t, α) ≥ 0, so that increasing α either increases or has no effect on the marginal product of capital.
Some of the energy produced (s(t), say) is used to increase the probability of survival from date t to date t + 1, given by σ(s(t)), for t = 1, … , T. Formally, this “survival function” σ: [0, ∞) → [0, 1] is continuous on [0, ∞) and continuously twice differentiable on (0, ∞). Further, σ′(s(t)) > 0 and σ"(s(t)) < 0, for all s(t) > 0, so that additional increases in survival become increasingly expensive as more energy is allocated to this purpose. “Corner solutions” are avoided by assuming that σ(0) = 0 and that σ′(s(t)) → ∞, as s(t) → 0, whereas σ(s(t)) → σ̄ ≤ 1 and σ′(s(t)) → 0, as s(t) → ∞. Let s denote the entire profile of the s(t), for t = 1, … , T.
Net energy supply in the model is determined by K and s as y(K, s) ≡ (y(K, s, 0), … , y(K, s, T)), where y(K, s, 0) = − C(K) and y(K, s, t) = F(K, t, α) − s(t), for t = 1, … , T. The overall probabilities of survival to each age are determined by s as p(s) ≡ (p(s, 0), … , p(s, T)), where p(s, 0) = 1, p(s, 1) = p̄ ∈ (0, 1], and p(s, t) = p̄Π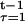 σ(s(τ)), for t = 2, … , T. Define then M ≡ {(p(s), y(K, s)), for K ≥ 0 and s ≥ 0} as the set of survival probability profiles and net energy profiles that are feasible in the model. Let p and y represent the profiles of survival probabilities and net energy, for t = 0, … , T, where dependence on K and s may be suppressed.
σ(s(τ)), for t = 2, … , T. Define then M ≡ {(p(s), y(K, s)), for K ≥ 0 and s ≥ 0} as the set of survival probability profiles and net energy profiles that are feasible in the model. Let p and y represent the profiles of survival probabilities and net energy, for t = 0, … , T, where dependence on K and s may be suppressed.
Growth Rate Maximization.
What steady-state population growth rate can be sustained in a society where the probability and net energy profiles are p and y? If this growth rate is r, a steady-state population has an age distribution in the proportions 1, p(1)e−r, p(2)e−2r, … , p(T)e−rT. A given profile of lifetime net energy supply is then economically feasible in such a steady state if the total deficit is not more than the total surplus, that is, if L(r, p, y) ≡ Σ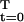 p(t)y(t)e−rt ≥ 0.
p(t)y(t)e−rt ≥ 0.
The Euler–Lotka equation can be obtained formally from this last inequality by requiring equality, setting y(0) = −1, supposing that y(t) ≥ 0, but not all zero, for t = 1, … , T, and treating y(t) ≥ 0 as expected offspring. The Euler–Lotka equation has a unique solution for r, the “rate of return” on an investment of one individual who is alive at time 1 and who generates a stream of expected offspring “repayments” y(t) ≥ 0 for t = 1, … , T. This rate of return is the steady-state growth rate achieved in the long-run steady state.
The approach here derives such rates of return, or steady-state growth rates, from a time pattern of net energy supply rather than fertility. Although the Euler–Lotka equation may be appropriate with no parental investment, the present approach is useful when such resource transfers from older to younger individuals are the basic determinant of population growth. The pattern of fertility is endogenously limited by the pattern of energy transfers in the sense that it generates the same steady-state growth rate, but it is otherwise unrestricted.
The issue is then to find the maximum growth rate possible in the present model, because a type enjoying this maximum rate would ultimately dominate. If the set of feasible growth rates is Q ≡ {r ∈ (−∞, ∞)|∃(p, y) ∈ M ϶ L(r, p, y) ≥ 0}, then:
Lemma 1.
r* = max{r|r Q} ∈ (−∞,∞) is well defined.
Proof:
See Appendix for this and other proofs (published as supporting information on the PNAS web site, www.pnas.org).
The “basic problem” of finding this maximum growth rate and the profiles that generate it can be recast as follows:
Proposition 1.
Suppose that r* is the maximum growth rate and p* and y* generate r*. It follows that these solve a “transformed problem” in that L(r*, p, y) ≤ L(r*, p*, y*) = 0 for all (p, y) ∈ M.
Proof:
If L(r*, p*, y*) > 0, growth rates greater than r* are possible given p* and y*. Similarly, if L(r*, p, y) > L(r*, p*, y*) = 0, then p and y generate growth rates greater than r*.
The overall logic of the present approach is as follows: We first maximize L(r, p, y) over (p, y) ∈ M, where r is an unknown parameter. Dynamic programming proves that this subsidiary problem has a unique solution (p*(r), y*(r)), say, for any r. The maximal growth rate, which exists by Lemma 1, must satisfy L(r*, p*(r*), y*(r*)) = 0, completing the characterization of the solution of the “basic problem” in terms of the “transformed problem.” Indeed, Proposition 2 shows that any solution to the transformed problem also solves the basic growth maximization problem.
The Optimal Life History.
The present section first characterizes the p* and y* that maximize L(r, p, y) ≡ Σ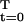 e−rt p(t)y(t), over (p, y) ∈ M. Using “dynamic programming,” it is necessary and sufficient that the optimal s satisfies the “backwards recursion” equations in the next paragraph.
e−rt p(t)y(t), over (p, y) ∈ M. Using “dynamic programming,” it is necessary and sufficient that the optimal s satisfies the “backwards recursion” equations in the next paragraph.
At date t = T − 1, … , 1 define V(K, t, α) = maxs(t){F(K, t, α) − s(t) + e−rσ(s(t))V(K, t + 1, α)} and set V(K, T, α) = F(K, T, α) at date T, because s(T) = 0, given T is the last period. V(K, t, α) is “value of life” at date t, because it is the expected discounted future lifetime energy surplus at date t, conditional on being alive at date t and given optimal investments in mortality reduction thereafter.
That is: first choose the optimal value of s(T − 1) at T − 1, yielding the optimal value of expected discounted net energy for that two-period problem as V(K, T − 1, α). The optimal value of s(T − 2) can now be obtained at T − 2, because V(K, T − 1, α) represents future expected payoffs. This process can be repeated, moving backward, finally obtaining V(K, 1, α) = maxs(1), … ,s(T−1) Σ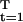 (Π
(Π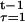 σ(s(τ))) (F(K, t, α) − s(t))e−r(t−1). It follows that p̄e−rV(K, 1, α) − C(K) = maxs L(r, p(s), y(K, s)) is the value of the objective in the last section, maximized only over the s(t), for t = 1, … , T.
σ(s(τ))) (F(K, t, α) − s(t))e−r(t−1). It follows that p̄e−rV(K, 1, α) − C(K) = maxs L(r, p(s), y(K, s)) is the value of the objective in the last section, maximized only over the s(t), for t = 1, … , T.
It follows from the definition of V(K, t, α) that the optimal s(t) are uniquely determined by σ′(s(t))V(K, t + 1, α)e−r = 1, for t = 1, … T − 1.e Thus, the marginal discounted benefit of the increased probability of surviving to the next date from a small increment in s(t), that is, σ′(s(t))V(K, t + 1, α)e−r, equals its marginal cost, which is just 1. The optimal profile of mortality is then linked to the value of life. Indeed, it can be shown that the present assumption that, other things being equal, output has an inverted U shape, implies that optimal mortality is U-shaped, as is true for most species.f
This analysis completes the solution to the current dynamic programming problem:
Lemma 2.
It follows that V(K, t, α) ≥ 0 is continuous in K ≥ 0, and that VK(K, t, α) > 0 and VKK(K, t, α) are continuous in K > 0, for any α ∈ A, and for t = 1, … , T. There is a K* > 0 maximizing p̄e−rV(K, 1, α) − C(K) and therefore satisfying p̄VK(K*, 1, α)e−r = C′(K*) and p̄VKK(K*, 1, α)e−r − C"(K*) ≤ 0.
Proof:
See Appendix.g
The first-order condition, p̄VK(K*, 1, α)e−r = C′(K*), is that the marginal discounted lifetime benefit of an increase in brain size equals its marginal cost.
Now let s*(r) denote the profile of s associated with K*(r), so that maxp,y∈M L(r, p, y) = L(r, p*(r), y*(r)), where p*(r) = p(s*(r)) and y*(r) = y(K*(r), s*(r)). By Proposition 1, the maximum growth rate r*, as in Lemma 1, is therefore attained uniquely by K*(r*) and s*(r*) and satisfies L(r*, p*(r*), y*(r*)) = 0. Conversely:
Proposition 2.
If L(r*, p*(r*), y*(r*)) = 0, for any growth rate r*, then maxp,y∈M L(r, p, y) < 0, for all r > r*, so K*(r*) and s*(r*) are the unique solutions of the basic growth rate maximization problem, and this maximum growth rate is r*.
Proof:
See Appendix.
Note that this formulation of production and mortality means that restricting attention to 1, … , T is without loss of generality. That is, suppose an additional range of ages with negative (or zero) production were included. It would then be optimal to choose s = 0 at date T, and the individual would die before any negative production occurred.h This apparently more general problem is then equivalent to that here. That is, the maximum age here is endogenously determined by the production function.i
The Coevolutionary Effect of a More Challenging Environment.
The average exponential growth rate of humanity over the past 2 million years must have been, as an arithmetic necessity, close to zero. For simplicity, assume then that r* = 0. That is, the production function F, cost function C, and survival function σ are now subjected to the additional restriction that the maximum growth rate they permit is zero. Although this restriction might seem fortuitous, it becomes compelling once these functions depend implicitly on population density as an additional argument.
It is further assumed that a small change in the parameter α affects the production function so that there is no effect on total expected production. This assumption implies that a maximum growth rate of zero is maintained by a small change in α:
Lemma 3.
The maximum growth rate in the model is a differentiable function of α, r*(α), say. If r*(ᾱ) = 0, at α = ᾱ, and Σ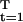 p*(t)Fα(K*, t, ᾱ) = 0, for p* = p(s*), then dr*(ᾱ)/dα = 0, so that a small change in α maintains zero growth.
p*(t)Fα(K*, t, ᾱ) = 0, for p* = p(s*), then dr*(ᾱ)/dα = 0, so that a small change in α maintains zero growth.
Proof:
See Appendix.
Because the growth rate is unaffected by a change in α, we are considering here the “pure” effect of learning, which is to shift the time path of energy production toward later ages.
The main theoretical results of the paper now follow:
Theorem 1.
(I) dK*/dα = p̄VKα(K*, 1, ᾱ)/[C"(K*) − p̄VKK(K*, 1, ᾱ)] > 0, so increasing α increases the optimal neural stock. (II) ds*(t)/dα = −σ′(s*(t))[Vα(K*, t + 1, ᾱ) + VK(K*, t + 1, ᾱ)(dK*/dα)]/σ"(s*(t))V(K*, t + 1, ᾱ) > 0, for t = 1, … T − 1, so that increasing α also increases optimal expenditures on survival.
Proof:
See Appendix.
That is, a more challenging environment, with its greater importance for learning, leads to coevolutionary increases in brain size and life expectancy.
Extensions.
Brain size and longevity coevolve here because of a common underlying cause: a more challenging environment. Such coevolution would be strengthened if survival were an increasing function of neural capital. If survival were σ(s(t), K), that is, mortality would automatically fall whenever K increased, other things being equal. If exogenous overall body size were an important determinant of survival, the survival function might then be σ(s(t), t), which generalization also raises no obvious difficulties. Indeed, if the survival function were σ(s(t)) + β, where β is an exogenous component of survival, an increase in β would increase K and all of the s(t), so brain size and longevity would then coevolve because of an alternative common cause.j
As a technical convenience, there was assumed to be no minimal level of s(t) below which the probability of survival would be zero. It was similarly assumed that survival at each age depends on expenditures at that age only and not on previous expenditures. Changing these two assumptions would make the model more realistic but might not change the main qualitative results. If survival were to depend on previous expenditures, for example, so that there was a “health” stock comparable to a brain stock, survival beyond the ages where production is negative and optimal s(t) is zero would be possible, as may be the case in the real world.
Empirical Evidence Supporting the Theory
Ecology, Brain Size, and Longevity Among Nonhuman Primates.
Our theory of primate evolution suggests the following predictions about extant nonhuman primates. The first is that dietary niche as captured by grade (prosimian, monkey, or ape) and other features of the ecology affecting the productivity of the brain, such as a large home range with its greater demands on spatial memory or a diet emphasizing ripe fruits (22), would entail an increased brain size, after controlling for body size. This prediction is confirmed in a multiple regression analysis of brain weight on grade, and the natural logarithms of range size, percent fruits in the diet, and body weight, using all 91 primate species for which such information is available (excluding humans; see supporting information at www.pnas.org for details on data sources and regression results). Grade is significant, with prosimians having smaller brains than monkeys (P < 0.0001), whose brains, in turn, are smaller than those of apes (P < 0.0001). Range size (P < 0.0005) and body weight (P < 0.0001) have significant positive effects, but percent fruit is not significant (P = 0.39). Second, our model suggests that a longer life span would be implied by an increased brain size, even after controlling for the feeding niche. This prediction is also confirmed when estimates of maximum life span are regressed on grade as a fixed effect (P = 0.02), predicted brain weight from the first regressionk (P = 0.007) and log body weight (P = 0.03). In fact, the partial effect of body weight is now negative. Third, larger-brained species would shift productivity to older ages and hence allocate more effort to survival. This prediction would be reflected in slower growth rates and later first reproduction. Age of first reproduction increases significantly as either predicted brain weight (P < 0.002) or maximum life span increase (P < 0.005), with body size controlled (P = 0.18), where overall R2 = 0.76.l
Comparisons of Hunter–Gatherers and Chimpanzees.
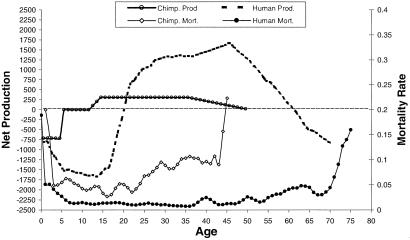
The most dramatic evidence in support of our theory arises from comparing the age profiles of mortality and production for wild chimpanzees with those for modern human hunter–gatherers (foragers) living under conditions similar to our evolutionary past. Fig. 1 shows probabilities of survival and net production by age. The chimpanzee net production curve shows three distinct phases. The first phase, to about age 5, is the period of complete to partial dependence on mother's milk and of negative net production. The second phase is independent juvenile growth, lasting until adulthood, during which net production is zero. The third phase is reproductive, during which females, but not males, produce a surplus of calories that they allocate to nursing.
Figure 1.
Net food production and mortality: human foragers and chimpanzees. On the left, the vertical axis is net production in calories per day and on the right is the mortality hazard per year, smoothed with a locally weighted 5-yr running average after age 5. Details on all sources and estimation procedures for both human and chimpanzee production and consumption data are in supporting information on the PNAS web site at www.pnas.org and in ref. 23.
Humans, in contrast, produce less than they consume for about 20 years. Net production becomes increasingly negative until about age 14 and then begins to climb. Net production in adult humans is much higher than in chimpanzees and peaks at a much older age, reflecting the payoff of long dependency. More precisely, human peak net production is about 1,750 calories per day, reached at about age 45. Among chimpanzee females, peak net production is only about 250 calories per day and, because fertility decreases with age, net productivity probably decreases throughout adulthood.
These differences in age profiles of production reflect the importance of skill in energy acquisition. Chimpanzees obtain an average of about 95% of their calories from foods that are readily gathered by hand, whereas foragers obtain an average of only 8% of their food energy in this way. In contrast, foragers obtained about 32% of their food energy from extracted resources (which are underground, in hard shells, or bear toxins that must be removed) and 60% from hunted resources. Chimpanzees obtain about 3% of their calories from extracted resources and about 2% from hunting mammals (23).
Collected resources require less learning, and both juvenile chimpanzees and human children obtain these resources efficiently. However, efficiency in extracting resources requires more learning. For example, the rate of root acquisition among Hiwi women does not asymptote until age 35–45 (23). Human hunting is the most skill-intensive foraging activity and differs qualitatively from hunting by other animals that typically either wait to ambush prey or use stealth-and-pursuit techniques. Human hunters use a wealth of information to make context-specific decisions during both the search and encounter phases of hunting. This hunting also involves much larger spatial areas than for chimpanzees. For example, Ache men aged 35 or more hunt in nearly 12,000 km2 of tropical forest in their lifetimes, whereas male chimpanzees cover only about 10 km2 (24). The forager hunting return rate (calories per hour hunting) quadruples from age 20 to age 35, peaking in the mid 30s.
Chimpanzee mortality is higher and rises more steeply with age than human mortality, which is more rectangular and rises only after about age 35. As a result, hunter–gatherer children also have a higher probability of survival to age 15 than do chimpanzees (60 vs. 35%). Chimpanzees spend less time as juveniles, with age of first birth for chimpanzee females being about 5 years earlier than for hunter–gatherer women. In natural habitats, chimpanzees have a much shorter adult life span than humans. At age 15, chimpanzee life expectancy is an additional 15 years, compared to 38 more years for human foragers. Women spend more than a third of their adult life in a postreproductive phase, whereas very few chimpanzee females survive to reach this phase. Less than 10% of chimpanzees survive to age 40, where human production peaks, but more than 15% of hunter–gatherers survive to age 70. By age 15, chimpanzees have consumed 43% and produced 40% of their expected lifetime calories, respectively; in contrast, by age 15, humans have consumed 22% and produced only 4% of their expected lifetime calories!
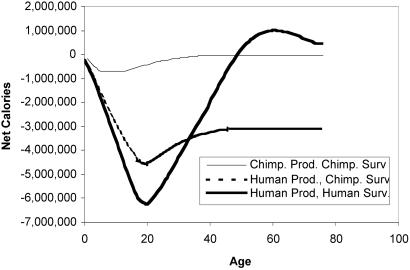
The relationship between survival rates and age profiles of production is made even clearer in Fig. 2, which plots net expected cumulative productivity as a function of age. This dependent variable involves multiplying the probability of being alive by the net productivity at each age and then cumulating over all ages up to the present. The long human training period is evident when the troughs in the human and chimpanzee curves are compared. The dashed line is a hypothetical cross of human production profiles with chimpanzee survival rates and shows that the human production profile would not be viable with chimpanzee survival rates, because expected lifetime net production would be negative.
Figure 2.
Cumulative expected net caloric production by age: humans and chimpanzees. Data sources are the same as those in Fig. 1.
Physiology and Genetics of Intelligence and Longevity.
The theory and data illuminate why natural selection exaggerated intelligence and longevity; the following evidence concerns how this actually happened. Most fundamentally, the grade shifts from monkeys to apes and from apes to humans are associated with differences in the lengths of the various phases of neural development (14). In early fetal development, humans spend 25 more days producing cortical neurons than do monkeys and apes. The greater proliferation of neurons in early fetal development has cascading effects, extending other phases of brain development and ultimately resulting in a larger, more complex and effective brain. Furthermore, in monkeys such as macaques, myelination of the brain begins prenatally and is largely complete in 3½ years, whereas in humans it continues to at least 12 years of age (25). Dendritic development is similarly extended to age 20 or beyond in humans. From a behavioral point of view, although cognitive development is largely complete among chimpanzees by about 8 years of age, formal abstract logical reasoning does not emerge in humans until age 16–18.
Research into brain aging and longevity suggests that some genes may have pleiotropic effects (influencing more than one trait) consistent with our model. The apolipoprotein E (apoE) allele system is a good example, because it seems to affect neurite growth and the aging of both the brain and the cardiovascular system. (The discussion here is based on ref. 26, which gives the original sources.) Brain aging is common in long-lived mammals. These signs of brain aging are delayed in humans relative to apes and in apes relative to monkeys. In humans, apoE has three common variants (apoE ɛ2, ɛ3, and ɛ4), whereas the great apes and other primates have apoE sequences most similar to human apoE ɛ4. Interestingly, this variant is a risk factor for both Alzheimer's disease and coronary artery disease, suggesting that the apoE ɛ2 and ɛ3 variants may have evolved to slow down both brain and cardiovascular aging. These other variants also promote neurite growth in cultured neurons, suggesting they also stimulate greater brain development and complexity. Although our understanding of the genetic bases of aging and brain development is incomplete, the apoE allele system is a candidate for a genetic basis of the coevolution predicted by our model.m
Discussion
Implications for Biology.
Here we apply capital theory to explain the coevolution of intelligence and longevity and the emergence of our species, but our results can also be applied to life history problems involving other capital stocks—physical size, for example. Life history theory typically utilizes the logic of capital investment theory; however, the link with the well-developed theory of capital investments in economics has never been fully exploited. Moreover, most recent life history analyses (27, 28) treat mortality rates as exogenous and derive aging, growth, and reproduction as a function of those rates. However, the assumption of exogeneity is clearly not realistic, because virtually all mortality hazards (e.g., predation, disease, and accidents) can be reduced with anatomical, physiological, and behavioral adaptations. Our approach allows both capital investments and expenditure on mortality reduction to be subject to natural selection and as such is capable of providing a deeper and more general understanding of life-span evolution.
The generality of the basic argument here can be illustrated as follows. In the first place, the effects of learning-intensive foraging strategies on brain size and life span are not limited to primates but may also arise in birds, for example. Parrots (Psittacidae) engage in a great deal of extractive foraging. They are also highly altricial (underdeveloped at birth and dependent on parental provisioning for long periods) and are extreme among birds in terms of both relative brain size and longevity (29). Terrestrial galliforms, such as quail, that simply peck at foods on the ground are precocious, much shorter lived (30), and smaller brained (29). In comparative studies, the degree of altriciality at birth, which tends to be associated with greater delays in foraging efficiency (31), is the best predictor of relative brain size, especially of hemisphere size as opposed to brain stem, optic lobes, or cerebellum (29). In addition, relative brain size is negatively associated with the rate of mortality increase with age among adults (32).
As a second example that ventures still further from brain evolution in primates, the present model of capital formation helps to explain the several-thousand-fold variation in insect life spans (ref. 33 and refs. therein). That is, it implies that life spans should also correlate with the productivity of nonneural capital. Eusocial insects typically make large energy allocations to building hives and mounds, to setting aside stores of energy, and in nurturing a large work force of nonreproductive individuals. All these energy allocations are highly productive investments, so that the total energy available for reproduction grows as the physical plant, stores, and work force grow. Such a strategy induces a shift in productivity toward older ages and hence favors increased survival and longevity. Indeed, queens in large eusocial societies are especially long-lived, sometimes surviving for several decades.n It has been argued that such extreme longevity is due to low “extrinsic” mortality (35), but this explanation is incomplete, because as the authors point out, ant colonies are heavily defended and fortified. Rather, low mortality is a coevolutionary response to conditions favoring large capital stocks.
Implications for Social, Behavioral, and Medical Sciences.
Much recent debate on the future of the human life span has focused on the hypothesis that survivorship is becoming rectangularized (i.e., the distribution of deaths is becoming increasingly compressed), with an upper limit of 85 ± 6 years (36) and diminishing effects of modern medical interventions. It is of interest then that comparisons between chimpanzees and hunter–gatherers (Fig. 1) suggest that “rectangularization” may have occurred over a long period and may not be only a response to modern medicine.
Furthermore, the life histories of hunter–gatherers are remarkably similar to those of people living in modern societies. Hunter–gatherer children are largely supported by their parents until about age 18 (when food production approximately equals consumption), after which time productivity rises steeply through the 20s until the mid-30s. The more skill intensive the task, the greater the delay to peak performance and the greater the increase in productivity with “on-the-job training” (23). High productivity is maintained until the 60s, when the effects of age become significant. This pattern of development and aging bears a striking resemblance to modern societies, where wages depend on education-based capital. This evolutionary legacy raises an important question about modern behavior. To what extent is this behavior determined by an evolved physiology? Such physiological processes may be genetically regulated and so change only slowly in response to natural selection. On the other hand, given that the point of the large human brain is to respond flexibly to environmental variation, to what extent has our psychology evolved to respond appropriately to changing conditions? For example, how does the correlation between education and longevity among people in Europe, the U.S., and Asia (37, 38) decompose into a long-term physiological effect and a short-term adaptive response mediated by health-related behaviors such as diet and exercise? Further theoretical models that integrate biological and economic analysis are necessary to answer such questions.
Supplementary Material
Acknowledgments
A.J.R. acknowledges support from the Social Sciences and Humanities Research Council of Canada and from a Canada Council Killam Research Fellowship. H.S.K. acknowledges support from National Institute on Aging Grant, AG15906. We also thank the editor and referees of PNAS for helpful comments.
Abbreviation
- apoE
apolipoprotein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
For simplicity, t̄ is independent of K.
This assumes that V(K, t + 1, α) > 0; if V(K, t + 1, α) ≤ 0, then s(t) = 0.
We prove this claim for a continuous-time version of the model in ref. 21. Simulations illustrating this claim are also available at www.unm.edu/∼hkaplan/pnas/regress.htm.
The optimal K is further assumed to be unique, and the second-order condition is strengthened to p̄VKK(K*, 1, α)e−r − C"(K*) < 0.
If σ′(0) were finite, it might be optimal to set s(t) = 0 earlier than this, where the value of life remains positive but is small enough.
Data such as that in Fig. 1 suggest that hunter–gatherers experience a final phase of life during which net production is negative. However, this phase is relatively short and might vanish if nonfood production were accounted for.
It is less clear how to maintain zero population growth now, however.
Because brain size is endogenous (it is the dependent variable in the first regression), the problem of simultaneity can be addressed by using predicted brain size in this second regression. Similar results are obtained, however, when measured values are used instead of predicted values.
Similar results are obtained by using path analysis and the method of independent contrasts to control for phylogenetic relationships (unpublished results available from H.S.K.).
Pleiotropic effects are not required here. That is, genes affecting only intelligence might coevolve with those affecting only longevity. However, the existence of suitable pleiotropic genes tends to validate the present approach, because it suggests that longevity and intelligence were typically subject to simultaneous evolutionary pressure.
In addition, at lower population densities, fire ant queens themselves grow larger before reproducing and live longer, producing more generations of workers (34).
References
- 1.Friedenthal H. Zentralbl Physiol. 1910;24:321–327. [Google Scholar]
- 2.Sacher G A. In: Ciba Foundation Colloquia on Ageing. Wolstenhome G E W, O'Connor M, editors. London: Churchill; 1959. pp. 115–133. [Google Scholar]
- 3.Sacher G A. In: Primate Functional Morphology and Evolution. Tuttle R, editor. The Hague: Mouton; 1975. pp. 417–441. [Google Scholar]
- 4.Economos A C. Gerontology. 1980;26:82–89. doi: 10.1159/000212399. [DOI] [PubMed] [Google Scholar]
- 5.Hakeem A, Sandoval G R, Jones M, Allman J. In: Handbook of the Psychology of Aging. Abeles R P, Catz M, Salthouse T T, editors. San Diego: Academic; 1996. pp. 78–104. [Google Scholar]
- 6.Dunbar R I M. Evol Anthropol. 1998;6:178–190. [Google Scholar]
- 7.Aiello L, Wheeler P. Curr Anthropol. 1995;36:199–221. [Google Scholar]
- 8.Martin R D. News Physiol Sci. 1996;11:149–156. [Google Scholar]
- 9.Allman J, McLaughlin T, Hakeem A. Proc Natl Acad Sci USA. 1993;90:118–122. doi: 10.1073/pnas.90.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose M R, Mueller L D. Am J Hum Biol. 1998;10:409–420. doi: 10.1002/(SICI)1520-6300(1998)10:4<409::AID-AJHB2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 11.Hofman M A. Quart Rev Biol. 1983;58:495–512. doi: 10.1086/413544. [DOI] [PubMed] [Google Scholar]
- 12.Gadgil M, Bossert W H. Am Nat. 1970;104:1–24. [Google Scholar]
- 13.Jerison H. Evolution of the Brain and Intelligence. New York: Academic; 1973. [Google Scholar]
- 14.Parker S T, McKinney M L. Origins of Intelligence: The Evolution of Cognitive Development in Monkeys, Apes and Humans. Baltimore: Johns Hopkins Univ. Press; 1999. [Google Scholar]
- 15.Fleagle J G. Primate Adaptation and Evolution. New York: Academic; 1999. [Google Scholar]
- 16.Holliday M A. In: Human Growth. Falker F, Tanner J M, editors. Vol. 2. New York: Plenum; 1978. pp. 117–139. [Google Scholar]
- 17.Austad S N, Fischer K E. Am J Primatol. 1992;28:251–261. doi: 10.1002/ajp.1350280403. [DOI] [PubMed] [Google Scholar]
- 18.Milton K. Sci Am. 1993;269:70–77. doi: 10.1038/scientificamerican0893-86. [DOI] [PubMed] [Google Scholar]
- 19.Byrne R W. In: Machiavellian Intelligence II: Extensions and Evaluations. Whiten A, Byrne R W, editors. Cambridge, U.K.: Cambridge Univ. Press; 1997. pp. 289–311. [Google Scholar]
- 20.Gibson K R. In: Primate Ontogeny, Cognition, and Social Behavior. Else J G, Lee P C, editors. Cambridge, U.K.: Cambridge Univ. Press; 1986. pp. 93–105. [Google Scholar]
- 21. Robson, A. J. & Kaplan, H. S. (2002) Am. Econ. Rev., in press. [DOI] [PubMed]
- 22.Clutton-Brock T H, Harvey P H. J Zool (London) 1980;109:309–323. [Google Scholar]
- 23.Kaplan H K, Hill K, Lancaster J B, Hurtado A M. Evol Anthropol. 2000;9:156–185. [Google Scholar]
- 24.Wrangham W R, Smuts B. J Reprod Fert Suppl. 1980;28:13–31. [PubMed] [Google Scholar]
- 25.Gibson K R. In: Brain Maturation and Cognitive Development: Comparative and Cross-Cultural Perspectives. Gibson K R, Peterson A C, editors. New York: Aldine de Gruyter; 1991. pp. 29–64. [Google Scholar]
- 26.Finch C E, Sapolsky R M. Neurobiol Aging. 1999;20:407–428. doi: 10.1016/s0197-4580(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 27.Charnov E L. Life History Invariants: Some Explanations of Symmetry in Evolutionary Ecology. Oxford, U.K.: Oxford Univ. Press; 1993. [Google Scholar]
- 28.Kirkwood T B L, Austad S N. Nature (London) 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- 29.Bennett P M, Harvey P H. J Zool (London) (A) 1985;207:151–169. [Google Scholar]
- 30.Ottinger M A. Exp Gerontol. 2001;36:859–868. doi: 10.1016/s0531-5565(00)00246-1. [DOI] [PubMed] [Google Scholar]
- 31.Ricklefs R E. Biol Rev. 1979;54:269–290. doi: 10.1111/j.1469-185x.1979.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 32.Ricklefs R, Scheuerlein A. Exp Gerontol. 2001;36:845–857. doi: 10.1016/s0531-5565(00)00245-x. [DOI] [PubMed] [Google Scholar]
- 33.Carey J R. Ann Rev Entomol. 2001;46:79–110. doi: 10.1146/annurev.ento.46.1.79. [DOI] [PubMed] [Google Scholar]
- 34.Ross K G, Keller L. Annu Rev Ecol Syst. 1995;26:631–656. [Google Scholar]
- 35.Keller L, Genoud M. Nature (London) 1997;389:958–1060. [Google Scholar]
- 36.Fries J F. N Engl J Med. 1980;303:130–136. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 37.Pappas G, Queen S, Haddem W, Fisher G. N Engl J Med. 1993;329:345–351. doi: 10.1056/NEJM199307083290207. [DOI] [PubMed] [Google Scholar]
- 38.Kunst A E, Mackenbach J P. Am J Pub Health. 1994;84:932–937. doi: 10.2105/ajph.84.6.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.