Abstract
The E7 protein of human papillomavirus type 16 was produced in Lactococcus lactis. Secretion allowed higher production yields than cytoplasmic production. In stationary phase, amounts of cytoplasmic E7 were reduced, while amounts of secreted E7 increased, suggesting a phase-dependent intracellular proteolysis. Fusion of E7 to the staphylococcal nuclease, a stable protein, resulted in a highly stable cytoplasmic protein. This work provides new candidates for development of viral screening systems and for oral vaccine against cervical cancer.
Infection with human papillomavirus type 16 (HPV-16) is the main factor associated with development of cervical cancer (42). The HPV-16 E6 and E7 proteins are constitutively produced in cervical carcinomas, and E7 was shown to interact with several cell compounds, causing deregulation of the cell cycle and cell transformation (43). E7 is a 98-amino-acid nuclear phosphoprotein that is devoid of any known enzymatic activity (36). In eukaryotic cells, E7's half-life is short (30 to 40 min); its degradation is mediated by the ubiquitin-proteasome pathway (31). E7 protein is widely studied because of its implication in carcinoma onset. It is also considered to be a good antigen candidate for the development of new vaccines against cervical cancer.
E7 production systems have been developed in both eukaryotes (1, 39) and prokaryotes (27, 34). Since the 1990s, several workers have investigated the use of bacteria as E7 antigen delivery vehicles to elicit an immune response against HPV-16 (14, 22). The gram-positive and generally regarded as safe (GRAS) commensal bacterium Streptococcus gordonii was used for this purpose to display E7 protein at the cell surface in fusion with export signals (30). These recombinant S. gordonii strains could elicit an immune response in mice and monkeys (23, 26). Although encouraging, these results rely on a commensal, GRAS but non-food-grade bacterium. One risk of commensal, and hence persistent, microorganisms is the induction of immunotolerance. Thus, a transient presentation of the antigen to the immune system by a noncommensal bacterium may be needed to avoid this risk.
None of the systems mentioned above seems to provide the combination of safety, sufficient yields, and simplified methods that would allow both purification and eventual oral immunization using E7. We therefore considered an alternative system for native E7 production based on a food-grade lactic acid bacterium. The best-known lactic acid bacterium, Lactococcus lactis, has been extensively engineered for the production of heterologous proteins (5, 6, 10, 18, 20, 21, 28, 29, 35, 37). Protein production in L. lactis offers advantages: L. lactis is a food-grade gram-positive bacterium that produces very low amounts of native exoproteins. It is therefore a good candidate for heterologous protein secretion in different applications ranging from industrial production of high-added-value proteins to in vivo use as a live vaccine. As L. lactis is a noncommensal and transient bacterium in the digestive tract (12), the risk of eliciting a tolerance response to a given antigen is diminished; furthermore L. lactis has already been used to produce a viral epitope (18) and a viral protein (7).
In this work, we used the nisin-inducible system (4, 16) to express the E7 gene in L. lactis. E7 synthesis was directed to cytoplasmic or extracellular locations. Both native and Nuc-fused E7 proteins were successfully expressed in both locations. Expression levels and stability of these proteins are reported under different growth conditions and in different genetic backgrounds. These studies show that E7 can be stably produced in either native or hybrid form under different growth conditions when exported from L. lactis.
Bacterial strains and plasmids and methods used
The bacterial strains and plasmids used in this work are listed in Table 1. L. lactis strains were grown in M17 medium (Difco) (38) supplemented with 1% glucose (GM17) or brain heart infusion (Difco) at 30°C without agitation. Escherichia coli was grown in Luria-Bertani (33) at 37°C. Unless otherwise indicated, plasmid constructions were first established in E. coli and then transferred to L. lactis by electrotransformation (17). Plasmids were selected by addition of antibiotics as follows (concentrations in micrograms per milliliter): for L. lactis, streptomycin (1,500), rifampin (50), erythromycin (5), chloramphenicol (10), and erythromycin and chloramphenicol together (2.5 and 5, respectively); for E. coli, ampicillin (100) and chloramphenicol (10). Plasmid DNA isolation and general procedures for DNA manipulations were essentially performed as described previously (33). PCR (apparatus from Perkin-Elmer Cetus, Norwalk, Conn.) was performed using Vent DNA Polymerase (Promega) and PCR sequences were confirmed using the Dye terminator sequencing kit (ABI PRISM BigDye Terminators; Applied Biosystems).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Replicon | Characteristic(s)a | Reference or source |
|---|---|---|---|
| Strains | |||
| E. coli TG1 | supE hsd Δ5 thi Δ(lac-proAB) F"(traD36 proAB-lacZΔM15) | 13 | |
| L. lactis MG1363 | Wild type, plasmid free | 11 | |
| L. lactis MG1363 clpP | Eryr, clpP disrupted by single-crossover recombination, plasmid free | 8 | |
| L. lactis MG1363 dnaK | Eryr, dnaK disrupted by single-crossover recombination, plasmid free | 15 | |
| L. lactis NZ9000 | MG1363 (nisRK genes into chromosome), plasmid free | 16 | |
| L. lactis NZ9000 clpP | Strr Rifr Eryr, MG1363 transconjugant carrying nisRK genes and clpP disruption, plasmid free | This work | |
| L. lactis NZ9000 dnaK | Strr Rifr Eryr, MG1363 transconjugant carrying nisRK genes and dnaK disruption, plasmid free | This work | |
| Plasmids | |||
| pBS SK II+ | (ColE1) | Apr | Stratagene |
| pVE8001 | (ColE1) | Apr, PCR fragment encoding trpA transcription terminator | I. Poquetb |
| pBS:NsiI:nuc | (ColE1) | Apr, PCR fragment, with NsiI sites at both extremities, encoding Nuc mature moiety | S. Nouailleb |
| pGEM-T | (ColE1) | Apr | Promega |
| pGEM-E7 | (ColE1) | Apr; PCR fragment encoding E7 | This work |
| pBS-E7 | (ColE1) | Apr; PCR fragment encoding E7 | This work |
| pCYT-Nuc | (pWV01) | Cmr; gene, expressed under PnisA encodes NucB mature moiety | 7 |
| pSEC-Nuc | (pWV01) | Cmr; gene, expressed under PnisA encodes SPUsp-NucB precursor | 7 |
| pCYT-E7 | (pWV01) | Cmr; gene, expressed under PnisA encodes the native E7 protein | This work |
| pSEC-E7 | (pWV01) | Cmr; gene, expressed under PnisA encodes SPUsp-E7 precursor | This work |
| pCYT-Nuc-E7 | (pWV01) | Cmr; gene, expressed under PnisA encodes a NucB-E7 fusion | This work |
| pSEC-Nuc-E7 | (pWV01) | Cmr; gene, expressed under PnisA encodes SPUsp-NucB-E7 precursor | This work |
For strains, genotypic and phenotypic characteristics are given; for plasmids, plasmid and cloned-cassette characteristics are given.
Unité de Recherches Laitières et Génétique Appliquée, Institut National de la Recherche Agronomique, Domaine de Vilvert, Jouy en Josas, France.
Induction for E7 expression was performed using nisin (1 ng/ml; Sigma) for a 1- or 3-h period as previously described (7). As E7 is labile (31), the protein sample preparation from L. lactis cultures was adapted to include protease inhibitors and mild precipitation procedures. Briefly, protein samples were prepared from 2 ml of cultures. Cell pellet and supernatant were treated separately, essentially as described (20). To inhibit proteolysis in supernatant samples, 1 mM phenylmethylsulfonyl fluoride and 10 mM dithiothreitol were added. Proteins were then precipitated by addition of 100 μl of 100% trichloroacetic acid, incubated 10 min on ice, and centrifuged 10 min at 17,500 × g at 4°C. For the cell fraction, TES-Lys buffer (25% sucrose, 1 mM EDTA, 50 mM Tris-HCl [pH 8.0], lysozyme [10 mg/ml]) was complemented with 1 mM phenylmethylsulfonyl fluoride and 10 mM dithiothreitol. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, and immunorevelation with anti-Nuc or anti-E7 antibodies were performed as described (20).
Cloning and inducible expression of E7 in L. lactis
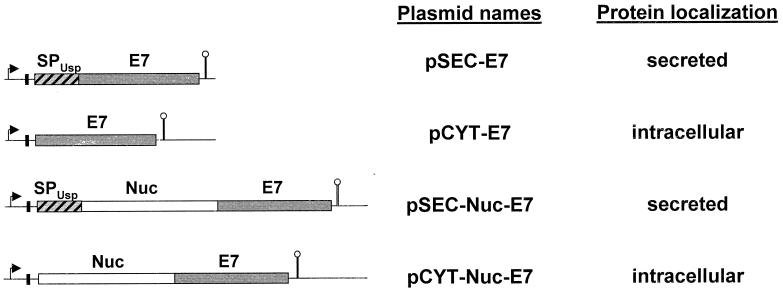
To achieve E7 production in different locations in L. lactis, several plasmids were constructed. The E7 gene was PCR amplified from vector pCDNA3-E7 (kindly provided by J. Alcocer, Laboratorio de Inmunología y Virología, Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, Nuevo Léon, Mexico). Primers used (and their sequences) were E7-HPV1 (5"-GATGCATCACAACATGGAGATACACCTACATTGCAT-3")for the coding strand and E7-HPV2 (5"-GGAGCTGTTATGGTTTCTGAGAACAGATGG-3") for the complementary strand. The PCR product (313 bp) was cloned into pGEM-T Easy Vector (Promega), resulting in pGEM-E7 plasmid (Table 1). The rho-independent transcription terminator trpA (3) was fused just downstream of the E7 gene by insertion of a SalI-ApaI fragment isolated from pGEM-E7 into the SalI-ApaI-cut pVE8001 vector (29). The resulting plasmid pBS-E7 was used for further constructions (Table 1). Two cassettes were constructed to produce HPV-16 E7 protein in cytoplasmic and secreted forms. An E7-trpA cassette was isolated from an EcoRV-NsiI-cut pBS-E7 and cloned into purified backbones isolated from EcoRV-NsiI-cut pCYT-Nuc and pSEC-Nuc (7, 32) resulting in pCYT-E7 and pSEC-E7 (Table 1; Fig. 1). In pSEC-E7, the E7 gene is fused in frame with a DNA fragment containing the ribosome binding site and the signal peptide of usp45 (SPUsp45), the gene encoding Usp45, the predominant L. lactis-secreted protein (Fig. 1) (40). In pCYT-E7, the fragment encoding SPUsp45 is absent. In both pSEC-E7 and pCYT-E7, expression of the E7 cassettes is controlled by the PnisA promoter (4). These plasmids were introduced into L. lactis strain NZ9000, which carries chromosomal copies of regulatory genes nisR and nisK (Table 1) (16).
FIG. 1.
Expression cassettes for E7 production and export in L. lactis. Schematic structures of native E7 or Nuc-E7 fusions (at left) expressed under control of the lactococcal PnisA promoter and carried by the indicated plasmids. For details of plasmid constructions, see the text and Table 1. Arrows indicate L. lactis promoter sequences of the nisin-inducible promoter (PnisA), solid vertical bars indicate the ribosome binding sites of the usp45 gene, striped bars indicate signal peptides of the usp45 gene; shaded bars indicate E7 coding region, open bars indicate NucB coding sequences, and stems topped with circles indicate transcription terminators (not to scale).
The secreted form of E7 is stably produced in L. lactis
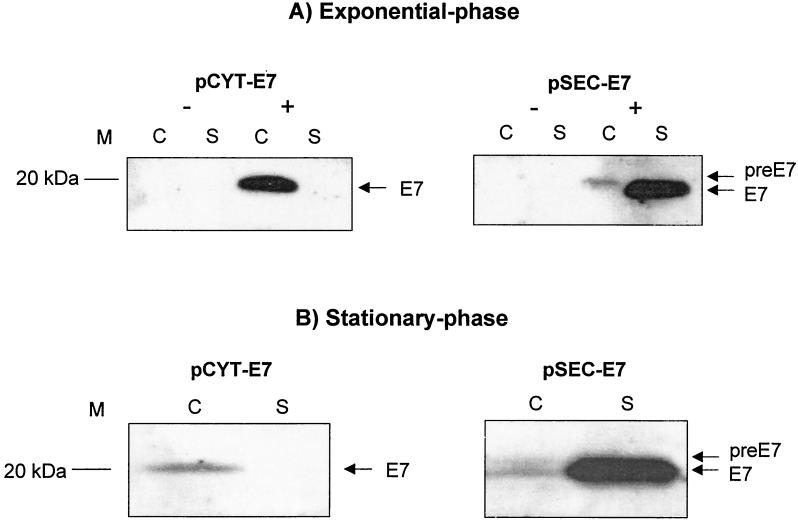
The capacity of L. lactis to accumulate E7 in either the cytoplasm or the extracellular medium was examined using strains NZ9000 containing pCYT-E7 and pSEC-E7, respectively (Table 1). Noninduced and induced culture samples were examined by Western blotting using anti-E7 antibodies. In the absence of nisin, no E7 signal was detected in either strain under different growth conditions. In late-exponential-phase (optical density at 600 nm [OD600] = 0.9 to 1.0) cells, induced NZ9000(pCYT-E7) cultures contained a distinct band in the cell fraction at the expected size for native E7 (19 kDa) (27), whereas no signal was detected in the supernatant (Fig. 2A). Similar analysis of NZ9000(pSEC-E7) resulted in two bands: (i) a weak band in the cell fraction corresponds to SPUsp-E7 precursor (preE7) (approximately 21 kDa) and (ii) a band in the supernatant fraction corresponds to the secreted mature E7 (Fig. 2A). E7 secretion appears to be efficient, as about 95% of the protein is detected in the supernatant. We estimate that E7 yield is about threefold higher for secreted protein than for cytoplasmic protein. We already observed this phenomenon in L. lactis for different proteins such as Nuc, rotavirus nonstructural protein 4 (NSP4) (7), Brucella abortus immunodominant antigen L7/L12 (32), or bovine β-lactoglobulin (2). Thus, in L. lactis, secretion seems the best strategy to achieve high production yields for eukaryotic (β-lactoglobulin), viral (E7, NSP4), or prokaryotic (L7/L12, Nuc) proteins regardless of their native locations. The above results suggest that proteins that are exported may avoid intracellular proteolysis.
FIG. 2.
Native E7 production in wt L. lactis depends on growth phase. E7 production and secretion were analyzed by Western blotting from cultures induced at different times such that 1 h after induction, the samples were harvested at exponential (OD600 ≈ 0.5 to 0.6) or stationary (OD600 ≈ 1.5) phase. Strains contain plasmid pCYT-E7 (encoding native E7, cytoplasmic form) or pSEC-E7 (encoding the precursor preE7, i.e., SPUsp45 fused to E7). (A) Exponential-phase cultures. Noninduced (−) and induced (+) cultures of wt L. lactis containing plasmids are as indicated. (B) Stationary-phase cultures. Arrows indicate positions of E7 mature and precursor forms. There is only a slight difference in migration positions of the preE7 (in cell lysates) and the secreted E7 mature form (in supernatant fraction). Abbreviations: C, cell lysates; S, supernatant fraction; M, positions and sizes of molecular mass markers.
Interestingly, analysis of protein samples extracted from stationary-phase (OD600 > 1) cultures of the above strains reveals a striking difference in E7 production: amounts of cytoplasmic E7 are markedly decreased, while amounts of secreted E7 are increased (Fig. 2B). We suggest that intracellular proteolytic degradation is greater in stationary growth phase and that the secreted protein can escape outside the cell via translocation.
We asked whether clpP and/or dnaK, factors known to be involved in intracellular protein degradation, also affect E7 turnover. ClpP is an ATP-dependent protease and the major cytoplasmic housekeeping protease in L. lactis (8), and the DnaK chaperone may promote proteolysis by maintaining misfolded proteins in a disaggregated state (41). To test whether ClpP and DnaK are involved in E7 degradation, we constructed an L. lactis NZ9000 strain harboring the clpP mutation (NZ9000 clpP) or dnaK mutation (NZ9000 dnaK) by conjugation using the following strategy: the donor strain was an erythromycin-resistant (Eryr) MG1363 clpP or MG1363 dnaK (kindly provided by H. Ingmer or K. Hammer, respectively) (8, 15). A spontaneous streptomycin-resistant (Strr) and rifampin-resistant (Rifr) strain was selected from NZ9000 and was used as the conjugation recipient. Conjugation was performed as described (17), and transconjugants were selected as triply Strr, Rifr, and Eryr. Chromosomal structure of the NZ9000 clpP or NZ9000 dnaK transconjugants was confirmed by PCR or Southern hybridization (data not shown). E7 production in NZ9000 clpP or NZ9000 dnaK containing plasmids pCYT-E7 or pSEC-E7 was analyzed by Western blot and compared to that of a wild-type (wt) strain. In exponential- and stationary-phase cultures, no significant differences in E7 patterns were observed between wt and clpP or dnaK strains: cytoplasmic E7 was equally degraded and secreted E7 yields were unchanged (data not shown).
Together, these results indicate that E7 intracellular proteolysis is ClpP and DnaK independent. Until recently, only two cytoplasmic proteases, ClpP and FtsH (8, 24), were identified in L. lactis. The existence of a third, as yet unidentified, protease was postulated by studies of a clpP mutant suppressor (9). E7 may thus be a useful screening target for identifying a putative L. lactis protease that, as suggested by our data, is activated in stationary phase.
Fusion of Nuc to the E7 N terminus stabilizes E7 production in L. lactis
Recent studies in our laboratory suggest that fusion of a protein of interest to Nuc could rescue and/or enhance production of a secreted heterologous protein in L. lactis, particularly when low yields are initially observed (32). Nuc is a well-characterized and stable protein that is resistant to denaturation and has readily detectable activity (19, 29). Furthermore, in a eukaryotic system, E7 is reportedly protected from proteolysis when epitope-tagged (Myc-tagged) at the N terminus (but not at the C terminus [31]). To test the effect of fusing Nuc to the N terminus of E7 (Nuc-E7), for both cytoplasmic and secreted forms, a nuc cassette harboring an NsiI restriction site at both extremities was purified from NsiI-cut pBS:NsiI:nuc (Table 1) (kindly provided by S. Nouaille, URLGA, INRA, Jouy en Josas, France) and cloned into NsiI-cut pSEC-E7 and pCYT-E7 backbones. The resulting plasmids, pCYT-Nuc-E7 and pSEC-Nuc-E7, were introduced into L. lactis NZ9000, and cytoplasmic and secreted Nuc-E7 production was then examined using these strains. Nuc-E7 production from induced exponential- and stationary-phase cultures was analyzed by Western blot experiments using anti-Nuc (data not shown) or anti-E7 antibodies (Fig. 3A).
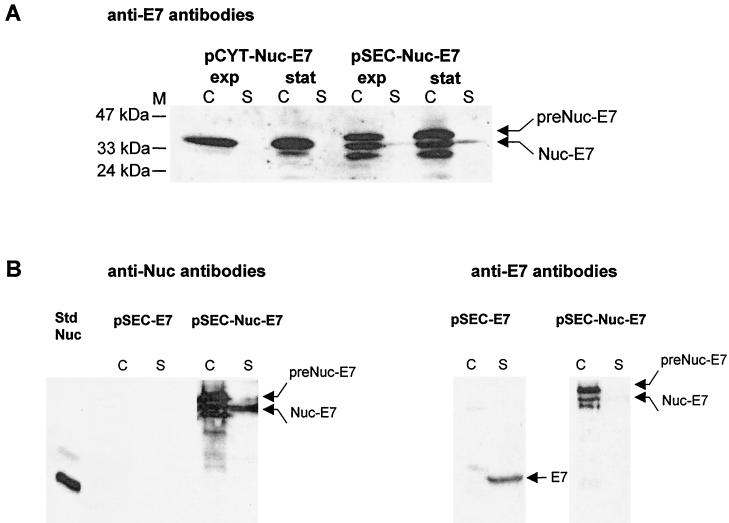
FIG. 3.
(A) Production of Nuc-E7 fusion is stable in L. lactis. Nuc-E7 production was analyzed by Western blot analysis using anti-E7 antibodies on exponential- and stationary-phase cultures of L. lactis strains containing pCYT-Nuc-E7 (encoding Nuc-E7) or pSEC-Nuc-E7 (encoding preNuc-E7). Arrows indicate positions of Nuc-E7 mature and precursor forms. Other bands in the supernatant fraction of L. lactis (pSEC-Nuc-E7) probably correspond to putative products resulting from secondary proteolytic cleavage of Nuc-E7. (B) Estimation of Nuc-E7 (pSEC-Nuc-E7) and native E7 (pSEC-E7) yields in L. lactis. Production yields were estimated for the secreted forms of both Nuc-E7 (pSEC-Nuc-E7) and native E7 (pSEC-E7). The left panel shows quantification of Nuc-E7 production by Western blot analysis using anti-Nuc antibodies. Nuc-E7 signal was compared to the signal of known amounts of commercial Nuc by quantitative scanning of blots after immunodetection (ImageQuant) (19). Total Nuc-E7 concentration is estimated to be around 15 μg/ml, of which about 3 to 5 μg/ml is in the supernatant. The right panel shows the analysis, after dehybridization, of the same membrane using anti-E7 antibodies. Signal intensities of native E7 forms were compared to those of Nuc-E7 forms in the panel at left. Total native E7 concentration is estimated to be about three times less than that of Nuc-E7. Abbreviations: C, cell lysates; S, supernatant fraction (note that culture supernatant samples were concentrated about fivefold prior to loading); M, positions and sizes of molecular weight markers; exp, exponential phase; stat, stationary phase; Std Nuc, NucA standard (50 μg/ml for std Nuc, i.e., 1 μg loaded on the gel; note that the faint higher band corresponds to NucB form enzyme).
For induced exponential- and stationary-phase cultures of NZ9000(pCYT-Nuc-E7), one major band, present in the cell fraction, is detected with anti-E7 antibodies. This band migrates at the expected size for a Nuc-E7 fusion (35 kDa). In the stationary-phase culture, a weak additional band is also present (Fig. 3A), which most likely corresponds to a Nuc-E7 degradation product. Thus, in contrast to results with the cytoplasmic form of native E7, the Nuc-E7 fusion accumulates in stationary-phase samples, suggesting that the Nuc moiety protects the E7 moiety from degradation.
For induced cultures of NZ9000(pSEC-Nuc-E7), Western blotting revealed three bands in the cell fraction in both exponential- and stationary-phase samples (Fig. 3A). The major upper band migrates at the expected size for the Nuc-E7 precursor (38 kDa). The two other bands comigrate with the intracellular forms found in NZ9000(pCYT-Nuc-E7) and correspond to mature Nuc-E7 and a putative cleavage product. In the supernatant, a single weak band is detected with anti-E7 antibodies, corresponding to the secreted Nuc-E7 fusion (Fig. 3A). In this case, secretion efficiency was only ∼10% (compared to ∼95% for native E7). Thus, exported Nuc-E7 protein remains cell associated, while exported E7 alone is released into the medium. Nuc activity plate assays performed on NZ9000(pSEC-Nuc-E7) showed a clear activity halo around colonies (data not shown), suggesting that Nuc-E7 is displayed on the cell surface (it was previously demonstrated that cytoplasmic Nuc forms give only a faint halo [19, 29]). To estimate amounts of Nuc-E7 versus E7 product, the same protein samples were analyzed using both anti-E7 and anti-Nuc antibodies (Fig. 3B). Nuc-E7 protein patterns were similar using both antibodies. To get an idea of the order of magnitude of E7 production yield in L. lactis, we estimated Nuc-E7 and E7 concentrations as follows. By comparison of Nuc-E7 signals with a Nuc standard loaded on the same gel (ImageQuant) (20), we estimated the quantity of total Nuc-E7 to be about 15 μg/ml (data not shown) (20). Using anti-E7 antibodies, total native E7 production was estimated to be about threefold lower than that of Nuc-E7. As purified E7 protein is not available, we used the Nuc-E7 protein concentration as determined by anti-Nuc antibodies as the standard; known amounts of Nuc-E7 were then used to estimate native E7 concentration (Fig. 3B). Using anti-E7 antibodies, we could then make an indirect estimation of native E7 concentration to be around 5 μg/ml (data not shown). These results show that despite greater amounts of total protein in the strain expressing pSEC-Nuc-E7, more E7 was secreted from the pSEC-E7 construction. Thus, secretion may be the system of choice to obtain stable native E7 production.
Interestingly, although pCYT-Nuc-E7 and pSEC-Nuc-E7 have an essentially identical design, the yield of exported Nuc-E7 protein is significantly greater (two- to threefold) than of the cytoplasmic form. These results are similar to those observed for native E7. We suggest that the secretion machinery may protect proteins from degradation by cytoplasmic proteases and thus account for the higher observed yields. Our results further show that the fusion of Nuc at the N terminus of E7 can stabilize E7 production in both cytoplasmic and secreted forms.
In summary, we successfully used L. lactis to produce HPV-16 E7, known as an extremely labile protein (31), in either the cytoplasm or the extracellular medium. Both these forms will be valuable in vaccine development trails. The amount of native E7 produced (estimated at 5 μg/ml for the secreted form) offers a promising starting point for E7 protein purification for physical and biochemical characterization, development of HPV screening assays and eventual production of purified vaccines, with essentially no risk of contamination with a toxic by-product.
The system developed in this study to produce HPV-16 E7 in L. lactis is interesting for the development of a new live vaccine against cervical cancer. Note that the use of the complete protein reportedly gives better interactions with the immune system components than that obtained with synthetic peptides (25). E7 was previously expressed at the S. gordonii surface and found to elicit an immune response in mice (23). While these results are promising, alternative E7 presentation systems will be useful in determining whether a totally innocuous and nonpersistent bacterium, L. lactis, can lead to development of a totally safe vaccine, with reduced risk of colonization or spread. In vivo immunogenicity tests using secreted and cytoplasmic forms of E7 for vaccination are now being developed in our laboratories.
Acknowledgments
We are grateful to Hanne Ingmer and Karin Hammer for providing clpP and dnaK mutant strains, respectively. We thank Juan Alcocer, Sébastian Nouaille, Isabelle Poquet, and Luciana Ribeiro for providing plasmid materials. We also thank Philippe Bouloc for valuable discussion of this work.
Yves Le Loir and Roberto Montes de Oca-Luna share credit in this work for senior authorship.
REFERENCES
- 1.Carter, J. J., N. Yaegashi, S. A. Jenison, and D. A. Galloway. 1991. Expression of human papillomavirus proteins in yeast Saccharomyces cerevisiae. Virology 182:513-521. [DOI] [PubMed] [Google Scholar]
- 2.Chatel, J.-M., P. Langella, K. Adel-Patient, J. Commissaire, J.-M. Wal, and G. Corthier. 2001. Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin. Clin. Diagn. Lab. Immunol. 8:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christie, G. E., P. J. Farnham, and T. Platt. 1981. Synthetic sites for transcription termination and a functional comparison with tryptophan operon termination sites in vitro. Proc. Natl. Acad. Sci. USA 78:4180-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vos, W. M. 1999. Gene expression systems for lactic acid bacteria. Curr. Opin. Microbiol. 2:289-295. [DOI] [PubMed] [Google Scholar]
- 6.Dieye, Y., S. Usai, F. Clier, A. Gruss, and J. C. Piard. 2001. Design of a protein targeting system for lactic acid bacteria. J. Bacteriol. 183:4157-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enouf, V., P. Langella, J. Commissaire, J. Cohen, and G. Corthier. 2001. Bovine rotavirus nonstructural protein 4 produced by Lactococcus lactis is antigenic and immunogenic. Appl. Environ. Microbiol. 67:1423-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frees, D., and H. Ingmer. 1999. ClpP participates in the degradation of misfolded protein in Lactococcus lactis. Mol. Microbiol. 31:79-87. [DOI] [PubMed] [Google Scholar]
- 9.Frees, D., P. Varmanen, and H. Ingmer. 2001. Inactivation of a gene that is highly conserved in Gram-positive bacteria stimulates degradation of non-native proteins and concomitantly increases stress tolerance in Lactococcus lactis. Mol. Microbiol. 41:93-105. [DOI] [PubMed] [Google Scholar]
- 10.Gaeng, S., S. Scherer, H. Neve, and M. J. Loessner. 2000. Gene cloning and expression of Listeria monocytogenes bacteriophage-lytic enzymes in Lactococcus lactis. Appl. Environ. Microbiol. 66:2951-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic acid streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geoffroy, M. C., C. Guyard, B. Quatannens, S. Pavan, M. Lange, and A. Mercenier. 2000. Use of green fluorescent protein to tag lactic acid bacterium strains under development as live vaccine vectors. Appl. Environ. Microbiol. 66:383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson, T. J. 1984. Ph.D. thesis. University of Cambridge, Cambridge, England.
- 14.Jabbar, I. A., G. J. Fernando, N. Saunders, A. Aldovini, R. Young, K. Malcolm, and I. H. Frazer. 2000. Immune responses induced by BCG recombinant for human papillomavirus L1 and E7 proteins. Vaccine 22:2444-2453. [DOI] [PubMed] [Google Scholar]
- 15.Koch, B., M. Kilstrup, F. K. Vogensen, and K. Hammer. 1998. Induced levels of heat shock proteins in a dnaK mutant of Lactococcus lactis. J. Bacteriol. 180:3873-3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuipers, O. P., P. G. de Ruyter, M. Kleerebezen, and W. M. de Vos. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15-21. [Google Scholar]
- 17.Langella, P., Y. Le Loir, S. D. Ehrlich, and A. Gruss. 1993. Efficient plasmid mobilization by pIP501 in Lactococcus lactis subsp. lactis. J. Bacteriol. 175:5806-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langella, P., and Y. Le Loir. 1999. Heterologous protein secretion in Lactococcus lactis: a novel antigen delivery system. Braz. J. Med. Biol. Res. 32:191-198. [DOI] [PubMed] [Google Scholar]
- 19.Le Loir, Y., A. Gruss, S. D. Ehrlich, and P. Langella. 1994. Direct screening of recombinants in gram-positive bacteria using the secreted staphylococcal nuclease as a reporter. J. Bacteriol. 176:5135-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Loir, Y., A. Gruss, S. D. Ehrlich, and P. Langella. 1998. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J. Bacteriol. 180:1895-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Loir, Y., S. Nouaille, J. Commissaire, L. Brétigny, A. Gruss, and P. Langella. 2001. Signal peptide and propeptide optimization for heterologous protein secretion in Lactococcus lactis. Appl. Environ. Microbiol. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodono, L. P., S. Chatfield, R. W. Tindle, K. Herd, X. M. Gao, I. Frazer, and G. Dougan. 1996. Immunization of mice using Salmonella typhymurium expressing human papillomavirus type 16 E7 epitopes inserted into hepatitis B virus core antigen. Vaccine 14:545-552. [DOI] [PubMed] [Google Scholar]
- 23.Medaglini, D., C. M. Rush, P. Sestini, and G. Pozzi. 1997. Commensal bacteria as vector for mucosal vaccines against sexually transmitted diseases: vaginal colonization with recombinant streptococci induces local and systemic antibodies in mice. Vaccine 15:1330-1337. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson, D., A. A. Lauridsen, T. Tomoyasu, and T. Ogura. 1994. A Lactococcus lactis gene encodes a membrane protein with putative ATPase activity that is homologous to the essential Escherichia coli ftsH gene product. Microbiology 140:2601-2610. [DOI] [PubMed] [Google Scholar]
- 25.Nindl, I., L. Gissman, S. G. Fisher, L. Benitez, J. Berumen, and M. Müller. 1996. The E7 protein of human papillomavirus (HPV) type 16 expressed by recombinant vaccinia virus can be used for detection of antibodies in sera from cervical cancer patients. J. Virol. Methods 62:81-85. [DOI] [PubMed] [Google Scholar]
- 26.Oggioni, M. R., R. Manganelli, M. Contorni, M. Tommasino, and G. Pozzi. 1995. Immunization of mice by oral colonization with live recombinant commensal streptococci. Vaccine 13:775-779. [DOI] [PubMed] [Google Scholar]
- 27.Pahel, G., A. Aulabaugh, S. A. Short, J. A. Barnes, G. R. Painter, P. Ray, and W. C. Phelps. 1993. Structural and functional characterization of the HPV16 E7 protein expressed in bacteria. J. Biol. Chem. 34:26018-26025. [PubMed] [Google Scholar]
- 28.Piard, J. C., R. Jimenez-Diaz, S. D. Ehrlich, V. A. Fischetti, and A. Gruss. 1997. The M6 protein of Streptococcus pyogenes and its potential as a tool to anchor biologically active molecules at the surface of lactic acid bacteria. Adv. Exp. Med. Biol. 418:545-550. [DOI] [PubMed] [Google Scholar]
- 29.Poquet, I., S. D. Ehrlich, and A. Gruss. 1998. An export-specific reporter designed for gram-positive bacteria: application to Lactococcus lactis. J. Bacteriol. 7:1904-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pozzi, G., M. Contorni, M. R. Oggioni, R. Manganelli, M. Tommasino, F. Cavalieri, and V. A. Fischetti. 1992. Delivery and expression of a heterologous antigen on the surface of streptococci. Infect. Immun. 60:1902-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reinstein, E., M. Scheffner, M. Oren, A. Ciechanover, and A. Schwartz. 2000. Degradation of the E7 human papillomavirus oncoprotein by the ubiquitin-proteasome system: targeting via ubiquitination of the N-terminal residue. Oncogene 19:5944-5950. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro, L. A., V. Azevedo, Y. Le Loir, S. C. Oliveira, Y. Dieye, J.-C. Piard, A. Gruss, and P. Langella. 2002. Production and targeting of the Brucella abortus immunodominant antigen L7/L12 by Lactococcus lactis: a first step towards food-grade live vaccines against brucellosis. Appl. Environ. Microbiol. 68:910-916. [DOI] [PMC free article] [PubMed]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 34.Sato, H., S. Watanabe, A. Furuno, and K. Yoshiike. 1989. Human papillomavirus type 16 E7 protein expressed in Escherichia coli and monkey COS-1 cells: immunofluorescence detection of the nuclear E7 protein. Virology 170:311-315. [DOI] [PubMed] [Google Scholar]
- 35.Savijoki, K., M. Kahala, and A. Palva. 1997. High-level heterologous protein production in Lactococcus and Lactobacillus using a new secretion system based on the Lactobacillus brevis S-layer signals. Gene 186:255-262. [DOI] [PubMed] [Google Scholar]
- 36.Smotkin, D., and F. O. Wettstein. 1986. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc. Natl. Acad. Sci. USA 83:4680-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steidler, L., K. Robinson, L. Chamberlain, K. M. Schofield, E. Remaut, R. W. Le Page, and J. M. Wells. 1998. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect. Immun. 66:3183-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Environ. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tommasino, M., M. Contorni, V. Scarlato, M. Bugnoli, K. Maundrell, and F. Cavalieri. 1990. Synthesis, phosphorylation, and nuclear localization of human papilloma-virus E7 protein in Schizosaccharomyces pombe. Gene 93:265-270. [DOI] [PubMed] [Google Scholar]
- 40.van Asseldonk, M., G. Rutten, M. Oteman, R. J. Siezen, W. M. de Vos, and G. Simons. 1990. Cloning, expression in Escherichia coli and characterization of usp45, a gene encoding a highly secreted protein from Lactococcus lactis MG1363. Gene 95:155-160. [DOI] [PubMed] [Google Scholar]
- 41.Wild, J., P. Rossmeissl, W. A. Walter, and C. A. Gross. 1996. Involvement of the DnaK-DnaJ-GrpE chaperone team in protein secretion in Escherichia coli. J. Bacteriol. 178:3608-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.zur Hausen, H. 1991. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 184:9-13. [DOI] [PubMed] [Google Scholar]
- 43.Zwerschke, W., and P. Jansen-Durr. 2000. Cell transformation by the E7 oncoprotein of human papillomavirus type 16: interactions with nuclear and cytoplasmic target proteins. Adv. Cancer Res. 78:1-29. [DOI] [PubMed] [Google Scholar]