Abstract
Thermus thermophilus HB27, an extremely thermophilic bacterium, exhibits high competence for natural transformation. To identify genes of the natural transformation machinery of T. thermophilus HB27, we performed homology searches in the partially completed T. thermophilus genomic sequence for conserved competence genes. These analyses resulted in the detection of 28 open reading frames (ORFs) exhibiting significant similarities to known competence proteins of gram-negative and gram-positive bacteria. Disruption of 15 selected potential competence genes led to the identification of 8 noncompetent mutants and one transformation-deficient mutant with a 100-fold reduced transformation frequency. One competence protein is similar to DprA of Haemophilus influenzae, seven are similar to type IV pilus proteins of Pseudomonas aeruginosa or Neisseria gonorrhoeae (PilM, PilN, PilO, PilQ, PilF, PilC, PilD), and another deduced protein (PilW) is similar to a protein of unknown function in Deinococcus radiodurans R1. Analysis of the piliation phenotype of T. thermophilus HB27 revealed the presence of single pilus structures on the surface of the wild-type cells, whereas the noncompetent pil mutants of Thermus, with the exception of the pilF mutant, were devoid of pilus structures. These results suggest that pili and natural transformation in T. thermophilus HB27 are functionally linked.
Thermus thermophilus HB27 is a gram-negative, yellow pigmented bacterium which exhibits high competence for natural transformation (27, 36). All species of the genus Thermus are extremely thermophilic. Members of the genus Thermus grow at temperatures ranging from 50 to 85°C with a temperature optimum of 70°C. In contrast to most of the extremely thermophilic bacteria, Thermus spp. grow under aerobic conditions (5). The genus Thermus is closely related to the genus Deinococcus, and phylogenetic studies of conserved genes suggest that these two lineages form a eubacterial phylum (5, 56).
The process of natural transformation can be divided into four discrete steps: competence induction, DNA binding, DNA uptake, and the heritable integration of incoming DNA or reconstitution of plasmid DNA. Proteins involved in uptake of DNA via natural transformation have been studied in several gram-negative bacteria, such as Neisseria gonorrhoeae (15, 51, 57), Acinetobacter sp. strain BD413 (14, 41), Haemophilus influenzae (9), Pseudomonas stutzeri (16, 17), Helicobacter pylori (1, 21, 48), and Synechocystis sp. strain PCC6803 (58), and some gram-positive bacteria, such as Bacillus subtilis and Streptococcus pneumoniae (6, 12, 39). A common feature of the transformation machineries is the implication of proteins exhibiting significant similarity to components of type IV pilus systems (12, 20). The only exception, to our knowledge, is the transformation system in H. pylori, whose known competence proteins (HP0333, ComH, and ComB) do not share any similarity with components of type IV pilus systems.
The significant similarities of competence proteins to proteins of type IV pilus systems lead to the fundamental question of whether type IV pili are involved in DNA uptake. This question has not been settled yet, but it seems to emerge that different bacteria might have different mechanisms.
Very little is known with respect to natural transformation systems in thermophiles and hyperthermophiles, although this means of lateral gene transfer probably had a very important impact on the evolution of life. In some scenarios, the universal tree of life is not rooted to an ancestral organism but to a pool or a net of ancestors. These ancestors are assumed to be hyperthermophiles with readily exchangeable genetic material. Moreover, there is substantial evidence for massive gene exchange between archaeal and bacterial hyperthermophiles resulting from genome-scale comparisons of these organisms (2, 8).
We chose T. thermophilus HB27 as a model organism to get insights into the transformation machinery of extremely thermophilic bacteria. In this study, we identified 28 putative competence genes in the partially complete genome sequence of T. thermophilus HB27. We report the identification of nine competence genes using gene disruption and transformation studies, and we present evidence for a link between systems for pilus synthesis and natural transformation in this organism.
MATERIALS AND METHODS
Strains, plasmids, and DNA manipulation.
T. thermophilus HB27 wild-type (DSM 7039) and mutant strains were grown in a 1:1 mixture of TM broth (27) and Luria-Bertani (LB) medium at 70°C. Antibiotics were added when appropriate (kanamycin, 20 to 40 μg/ml; ampicillin, 100 μg/ml; and streptomycin, 100 to 500 μg/ml). Escherichia coli strains were cultured at 37°C in LB medium. The molecular and genetic procedures were standard techniques. Southern hybridization experiments were performed as described previously (40).
DNA sequence analysis.
The nearly complete genomic sequence of T. thermophilus HB27 was determined by a whole-genome shotgun approach by the Göttingen Genomics Laboratory (G2L). Clones carrying HB27 genomic DNA of approximately 2.0 kb in length from small insert libraries representative of the whole genome were sequenced from both ends using LICOR IL-4200 and ABI PRISM 377 DNA sequencers. The generated sequence readings were assembled into contigs with the Prap software implemented in the STADEN software package. Sequence data were analyzed with BLAST programs of the National Center for Biotechnology Information database, the software package (version 10.0) of the Genetics Computer Group (University of Wisconsin Biotechnology Center), and the WIT platform (Integrated Genomics).
Generation of Thermus mutants.
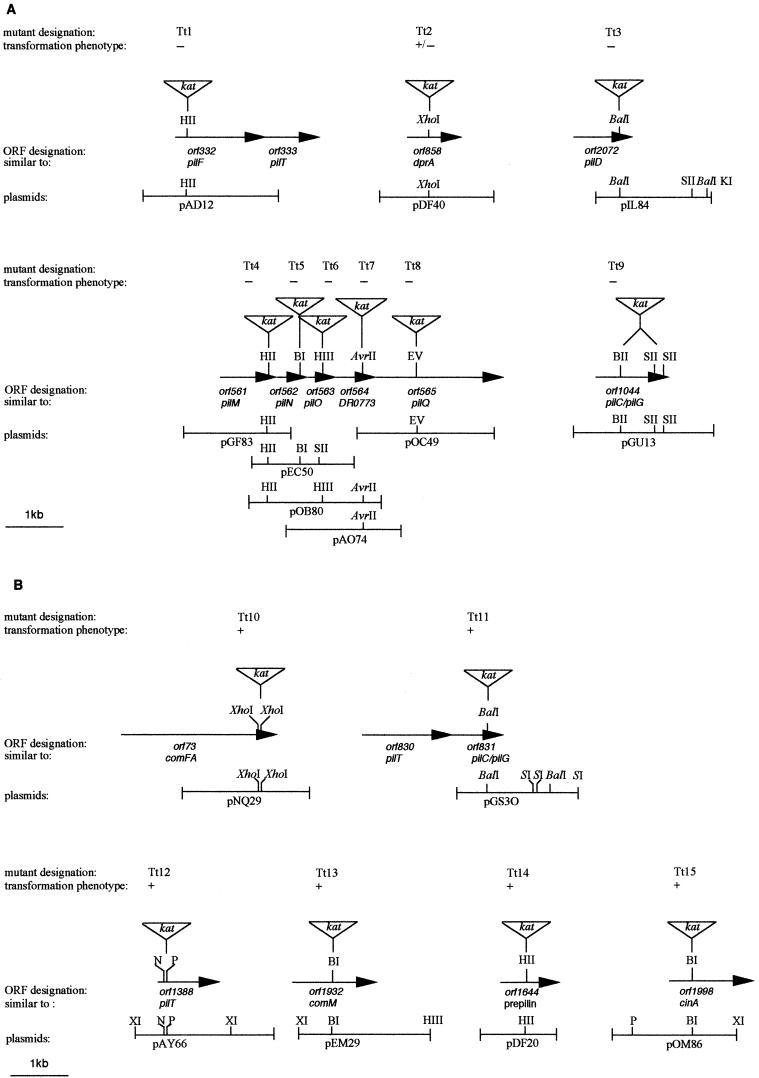
To analyze the role of potential competence genes, mutants were disrupted by a kanamycin resistance marker (kat) derived from the E. coli/T. thermophilus shuttle vector pMK18 (7). For gene disruption, recombinant plasmids of the Thermus gene library were used. Based on the sequence information, 15 gene library plasmids covering 11 different loci were selected (Fig. 1). The kat gene was inserted into unique sites of orf332, orf858, orf561, orf564, and orf565 or by substitution of distinct DNA fragments within orf73 and orf1044 by the kat gene (Fig. 1). To allow insertion of the kat gene into unique cleavage sites within the putative competence genes orf562, orf563, orf1388, orf1932, and orf1998, cleavage sites within the multiple cloning site had to be eliminated by subcloning into another vector. Therefore, the inserts of pEC50, pOB80, pAY66, pEM29, and pOM86 (Fig. 1) used for disruption of orf562, orf563, orf1388, orf1932, and orf1998, respectively, were subcloned into pBluescriptII KS/SK (Stratagene).
FIG.1.
Structural organization and gene disruption strategy of conserved ORFs within potential competence loci in the genome of T. thermophilus HB27. Physical map of mutant loci that are involved (A) and not involved (B) in natural transformation. In the restriction maps of gene bank plasmids covering different conserved ORFs of the potential competence loci, only selected restriction sites are shown. The triangle indicating the kat gene denotes the insertion site of the Kmr marker gene. The arrows denote direction of transcription. +, wild-type transformation frequencies; ±, 100-fold-reduced transformation frequencies; −, not transformable. BI, BamHI; BII, BglII; EV, EcoRV; HII, HincII; HIII, HindIII; N, NcoI; P, PstI; XI, XbaI.
To allow insertion of the kat gene into the BalI site of orf2072 or orf831, the BalI sites present in the flanking DNA regions had to be eliminated. Therefore, pIL84 and pGS30 (Fig. 1) were digested with SacII/KpnI and SacI, respectively, which was followed by treatment with Klenow enzyme and religation. This cloning strategy resulted in deletion of BalI restriction sites in the flanking DNA regions and allowed marker insertion into unique BalI sites in orf831 and orf2072, respectively.
The plasmids carrying the disrupted conserved open reading frames (ORFs) were transformed into E. coli DH5α, and transformants were selected on LB medium containing 100 μg of ampicillin/ml and 20 μg of kanamycin/ml. Plasmids were prepared, and the inserts were purified and transformed into T. thermophilus HB27 by natural transformation. Thermus transformants were selected on TM medium containing 40 μg of kanamycin/ml. The correct allelic replacement of chromosomal wild-type DNA by disrupted ORFs was verified by Southern hybridization.
Transformation studies.
For transformation of T. thermophilus HB27, a modified protocol of Koyama et al. (27) was used. For transformation studies with T. thermophilus HB27, spontaneous streptomycin-resistant mutants were selected by plating 108 cells on TM medium containing streptomycin (500 μg/ml). The genomic DNA of one selected streptomycin-resistant mutant was isolated and used as donor DNA for transformation studies. To enable parallel analyses of several Thermus mutants, a rapid transformation test system was established. One colony of a Thermus mutant, disrupted in one of the potential competence genes, was mixed with 20 μl of chromosomal DNA (100 ng/μl) of the streptomycin-resistant Thermus strain, plated on TM agar, and incubated for at least 8 h at 70°C to allow expression of the streptomycin marker. To select for transformants that had acquired the genes mediating the streptomycin resistance phenotype, the cells were plated on TM agar containing 100 μg of streptomycin/ml and subsequently incubated at 70°C overnight. The T. thermophilus HB27 wild-type strain was used as a transformation control.
Electron microscopy.
Thermus wild-type and mutant strains grown overnight on freshly prepared TM plates were negatively stained with 4% (wt/vol) uranylacetate. After drying on Formvar-coated copper grids, the cells were viewed with a Philips model EM301 transmission electron microscope at 80 kV.
Nucleotide sequence accession number.
The sequence data have been submitted to the GenBank database and the accession numbers are listed below in Table 2.
TABLE 2.
Identified competence genes in T. thermophilus HB27
| Annotation (accession no.) | bp | Molecular mass (kDa) | Mutant phenotype | Proposed function |
|---|---|---|---|---|
| dprA (orf858) (AF439555) | 1,005 | 36 | Transformation deficient | DNA translocation or recombination |
| pilM (orf561) (AF436067) | 1,134 | 41 | Noncompetent, nonpiliated | DNA transformation and assembly of pili |
| pilN (orf562) (AF436067) | 624 | 23 | Noncompetent, nonpiliated | DNA transformation and assembly of pili |
| pilO (orf563) (AF436067) | 582 | 21 | Noncompetent, nonpiliated | DNA transformation and assembly of pili |
| pilW (orf564) (AF436067) | 879 | 30 | Noncompetent, nonpiliated | DNA transformation and assembly of pili |
| pilQ (orf565) (AF436067) | 2,274 | 83 | Noncompetent, nonpiliated | Secretin-like competence protein |
| pilF (orf332) (AF436070) | 1,695 | 62 | Noncompetent, piliated | ATP-dependent function in assembly of the transformation apparatus |
| pilC (orf1044) (AF436068) | 1,326 | 48 | Noncompetent, nonpiliated | IMa protein involved in assembly of pili and the transformation apparatus |
| pilD (orf2072) (AF436069) | 1,065 | 38 | Noncompetent, nonpiliated | Prepilin peptidase involved in processing of prepilins |
IM, inner membrane.
RESULTS
Identification of competence genes by gene disruption.
Analysis of the transformation machineries of gram-positive and gram-negative bacteria have shown that DNA transfer systems often comprise homologues of type IV pilus biogenesis factors or homologues of proteins implicated in protein secretion machineries. Similarity searches within the Thermus genomic database with known proteins of type IV pilus biogenesis led to the identification of 24 homologues of type IV pilus biogenesis systems (Table 1). The deduced proteins of two other potential competence genes are similar to proteins of DNA translocation machineries, such as DprA and ComFA of H. influenzae and B. subtilis, respectively (Table 1). The deduced products of two more potential competence genes show similarities to proteins implicated in recombination of the incoming DNA with genomic DNA in H. influenzae and S. pneumoniae (Table 1).
TABLE 1.
Putative competence genes identified via homology searches in the genome sequence of T. thermophilus HB27
| Group and Thermus ORF(s) | Similar competence protein | Reference | % Amino acid similaritya and conserved motifsb |
|---|---|---|---|
| Group I (DNA translocation) | |||
| orf858 | DprA (H.i.)c | 25 | 43 |
| orf73 | ComFA (B.s.) | 29 | ATP binding motif |
| Group II (type IV pili) | |||
| orf561 | PilM (P.a) | 32 | 42 |
| orf562 | PilN (P.a) | 32 | —b |
| orf563 | PilO (P.a.) | 32 | —b |
| orf564 | Hypothetical protein DR0773 (D.r.) | 56 | 32 |
| orf565 | PilQ (P.a) | 31 | 35 |
| orf332 | PilF (N.g.) | 13 | 49 |
| orf333 | PilT (P.a.) | 55 | 59 |
| orf830 | PilB (P.a.) | 35 | 47 |
| orf1388 | PilT (P.a.) | 55 | 53 |
| orf2072 | PilD (P.a.) | 35 | 44 |
| orf1044 | PilC (P.a.) | 35 | 52 |
| orf831 | PilC (P.a) | 35 | 48 |
| orf822, orf824-825, orf827-828, orf1644-1646, orf1695-1697, orf1699 | Prepilins | 12 | Prepilin cleavage motif |
| Group III (recombination) | |||
| orf1932 | ComM (H.i.) | 19 | 57 |
| orf1998 | CinA (S.p.) | 38 | 46 |
The amino acid sequence similarities were calculated by comparing the deduced protein of the conserved ORFs in Thermus with the protein products of the prominent similar genes listed in Table 2.
Due to direct sequence comparison.
Strain designation: B.s., B. subtilis; D.r., D. radiodurans R1; H.i., H. influenzae; N.g., N. gonorrhoeae; P.a., P. aeruginosa; S.p., S. pneumoniae.
To determine whether the conserved potential competence genes are essential for transformation, 15 ORFs (Fig. 1), distributed over 11 conserved loci, were selected for gene disruption studies by use of a kanamycin resistance gene, kat. As indicated in Fig. 1A, the potential competence locus comprising orf561, orf562, orf563, orf564, and orf565 was subjected to single gene disruptions. The resulting mutants, Tt4 (orf561::kat), Tt5 (orf562::kat), Tt6 (orf563::kat), Tt7 (orf564::kat), and Tt8 (orf565::kat) were found to be completely noncompetent (Fig. 1A and Table 2). Due to the similarities of the deduced Thermus proteins to Neisseria type IV pilus proteins with the noncompetent mutant phenotypes, the Thermus ORFs orf561, orf562, orf563, and orf565 will be referred to hereafter as pilM, pilN, pilO, and pilQ (Table 1). Since orf564 is also a member of this competence gene cluster, although its deduced protein does not show any similarities to type IV pilus proteins, it was designated pilW. Due to the tandem arrangement of the pil genes within this Thermus competence locus and the analogous orientation, it cannot be excluded that a disruption of upstream-located genes results in a polar effect on downstream-located genes. However, since the ORFs located immediately downstream of pilQ encode key enzymes of tryptophan biosynthesis, a highly conserved chorismate synthase, and a shikimate kinase, a role for these genes in natural transformation can be excluded. This finding shows that at least pilQ, the last gene of this competence locus (Fig. 1A), is essential for natural transformation.
Three potential competence loci are comprised of genes whose deduced proteins show similarities to the type IV pilus factor PilT. To address the question of a role for the three pilT loci in natural transformation, single ORFs for each of these loci, orf332, orf1388, and orf831 (Fig. 1), were subjected to gene disruption. (Fig. 1). Analyses of the transformation phenotypes of the resulting mutants revealed that the natural transformation phenotype is completely abolished in mutant Tt1 (orf332::kat), whereas Tt11 (orf831::kat) and Tt12 (orf1388::kat) exhibit wild-type transformation frequencies (Table 2; Fig. 1). From these results, we conclude that orf831 and orf1388 are not implicated in natural transformation. Downstream of orf332, an ORF designated orf333 is present whose deduced protein shows similarities to PilT in N. gonorrhoeae and P. aeruginosa. Thus, it cannot be excluded that the noncompetent phenotype of the orf332 mutant is due to a polar effect on orf333 (Table 2; Fig. 1). However, since orf333 is flanked by the known T. thermophilus gene encoding a glutamyl-tRNA-amidotransferase (3), a polar effect on potential competence genes located downstream of orf332 and orf333 can be excluded.
The deduced protein of orf1044 is similar to the Pseudomonas PilC and the Neisseria PilG proteins (35, 50). Disruption of this ORF, designated pilC, resulted in the noncompetent mutant Tt9 (Fig. 1; Table 2). Since the ORF located immediately downstream of orf1044 is transcribed in the opposite direction, this finding clearly shows that orf1044 is essential for natural transformation.
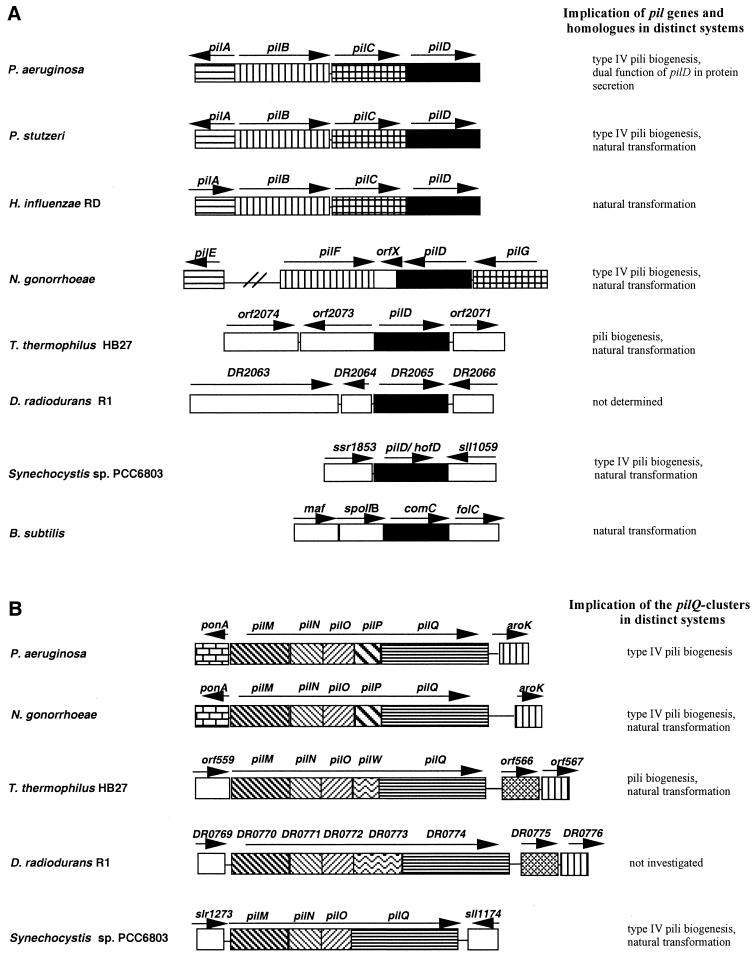
Disruption of the pilD-like orf2072 (Table 1) resulted in mutant Tt3 (Fig. 1A), which was found to be noncompetent (Table 2). It is interesting that the Thermus pilD-like gene, in contrast to pilD genes in other proteobacteria, such as P. aeruginosa, P. stutzeri, H. influenzae, and N. gonorrhoea, is not clustered with other pil genes (Fig. 2A). PilD homologues are also present in nonproteobacteria such as D. radiodurans, Synechocystis strain PCC6803, and B. subtilis (4, 34, 56, 58). Interestingly, the pilD-like genes in these transformable phylogenetically distant nonproteobacteria are, analogously to the Thermus pilD-like gene, not associated with other pil genes (Fig. 2A). The differences in the organization of the pilD-like gene in Thermus and pilD homologues in the genomes of transformable proteobacteria (Fig. 2A) might reflect potential distinct horizontal gene transfer events or internal recombination events in Thermus.
FIG. 2.
(A) Comparison of the pilD locus in different gram-negative proteobacteria and nonproteobacteria. orf2073 encodes a thermostable carboxypeptidase and orf2071 encodes a hypothetical protein in Thermus. ssr1853 encodes a hypothetical Synechocystis protein, and sll1059 encodes an adenylate cyclase. DR2065 is a pilD homologue in Deinococcus. The Deinococcus ORFs DR2063, DR2064, and DR2066 encode a polynucleotide phosphorylase, a hypothetical 17.3-kDa protein, and a conserved hypothetical protein, respectively. comC is a pilD homologue in B. subtilis. spoIIB and folC encode a sporulation factor and the enzyme folypoly-γ-glutamate synthetase-dihydrofolate synthetase, respectively. (B) Comparison of the genetic organization of the pilQ cluster in different gram-negative proteobacteria and nonproteobacteria. The Thermus ORFs orf559, orf566, and orf567 encode isopropylmalate dehydrogenase, chorismate synthase, and shikimate kinase, respectively. DR0775 is an aroC homologue and DR0776 is an aroK homologue in Deinococcus. slr1273 and sll1174 are predicted to encode hypothetical proteins in Synechocystis.
To address the question of a role of prepilin-like proteins in natural transformation, orf1644 was disrupted (Table 1; Fig. 1B). The resulting mutant Tt14 shows wild-type transformation frequencies (Fig. 1B), which leads to the conclusion that the prepilin-like ORF, orf1644, is not implicated in transformation.
Disruption of the H. influenzae dprA homologue orf858 resulted in mutant Tt2, which was found to exhibit a 100-fold reduced transformation frequency of 5 × 10−5 transformants/viable count (Fig. 1A; Table 2), whereas the wild type exhibited transformation frequencies of 6 × 10−3. Since the flanking ORF downstream of the dprA-like gene is orientated in the opposite direction, this result provides clear evidence that orf858 is essential for natural transformation of T. thermophilus HB27.
The disruption of the three additional selected ORFs, orf73, orf1932, and orf1998 (Fig. 1B), resulted in mutants exhibiting wild-type transformation frequencies, which leads to the conclusion that these ORFs are not implicated in the natural transformation of T. thermophilus.
Piliation phenotypes of Thermus wild-type cells and noncompetent mutants.
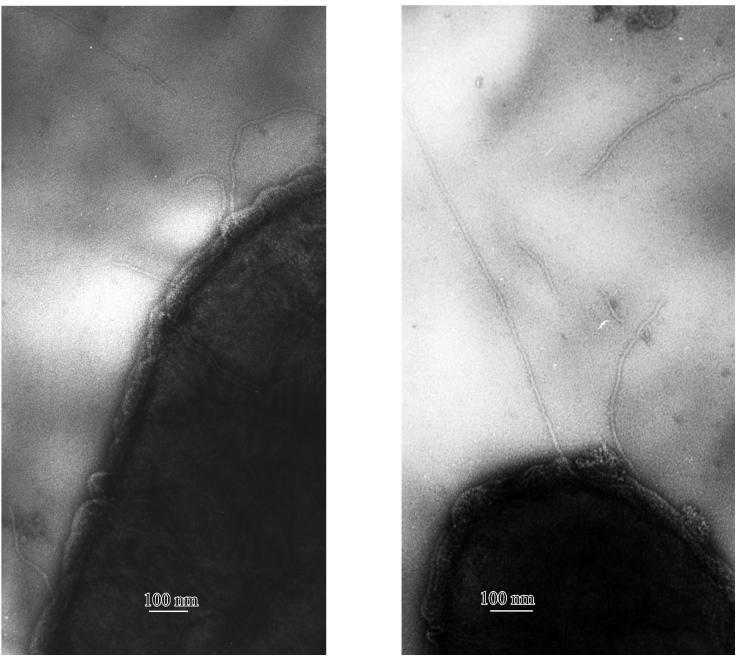
Electron microscopic studies of T. thermophilus HB27 cells led to the identification of individual pilus structures on the cell surface that were 6 nm in diameter and 1 to 3 μm in length (Fig. 3).
FIG. 3.
Representative sample showing the pilus structures on the surface of T. thermophilus HB27 wild-type cells. Electron microscopic investigations were conducted with uranylacetate-stained cells.
The significant similarities of the identified Thermus competence proteins to components of the type IV pilus systems, together with the presence of pilus structures on the cell surface of T. thermophilus HB27, led to the question of whether the transformation system is linked to the pilus biogenesis system in Thermus. To answer this question, noncompetent Thermus mutants disrupted in the competence genes pilD, pilC, and pilF or in individual genes of the pilM-pilQ cluster were analyzed with respect to their piliation phenotype. The pilC and pilD mutants and the pilM-pilQ mutants were found to be devoid of pilus structures (Table 2). This finding suggests either that transformation and pili are linked systems or that pili are implicated in transformation. The pilF mutant was found to exhibit pilus structures indistinguishable from those of the HB27 wild type. From these findings, we conclude that PilD, PilC, PilM, PilN, PilO, PilW, and PilQ but not PilF have a dual function in transformation and pilus biogenesis.
DISCUSSION
Detection of potential competence genes in the T. thermophilus genome database.
Homology searches in the genome sequence database of T. thermophilus HB27 led to the identification of 28 conserved ORFs whose protein products are similar to proteins involved in natural transformation of different gram-negative and gram-positive bacteria and to components of type IV pilus systems (Table 1). The deduced protein products of the potential competence genes show similarities to components of three major functional systems: (i) DNA translocation, (ii) type IV pilus biogenesis, and (iii) DNA recombination (Table 1).
Genetic organization of competence genes.
The genes of the pil competence locus in Thermus, comprising pilM, pilN, pilO, pilW (DR0773), and pilQ (Fig. 1A), are analogously orientated and tightly clustered, such that all adjacent genes have overlapping stop and start codons. This Thermus locus is related to pilMNOPQ loci implicated in type IV pilus biogenesis in P. aeruginosa and in pilus biogenesis and natural transformation in N. gonorrhoeae (10, 31, 32). In N. gonorrhoeae and P. aeruginosa, the pilMNOPQ cluster is preceded by ponA, which encodes a penicillin-binding protein (33, 46). Downstream of the Neisseria and Pseudomonas pilMNOPQ cluster is aroK, a shikimate kinase gene (31, 37). In contrast to this organization, there is no ponA homologue in the close vicinity of the conserved pilM-Q cluster of T. thermophilus, Synechocystis strain PCC6803, and D. radiodurans (Fig. 2B). aroK homologues are present downstream of the conserved pilM-Q cluster in the Thermus-Deinococcus group and are separated from the conserved pilM-Q cluster by a chorismate synthase gene. No aroK homologue was found in the close vicinity of the pil cluster in Synechocystis. The analogous organization of the genes within the conserved pilM-Q cluster in Thermus, Synechocystis, Pseudomonas, and Neisseria suggests that this conserved pil module in these distantly related bacteria has been acquired via horizontal gene transfer; and the differences in the flanking DNA regions might be due to an integration of the complete pil module in different genomic loci of the host genomes. The protein product of the fourth gene within the Thermus pilM-Q cluster, designated pilW, is similar to the hypothetical protein of D. radiodurans R1, encoded by the fourth ORF, DR0773, of the putative pilM-Q cluster in D. radiodurans. In P. aeruginosa and Neisseria, the fourth gene of the conserved gene cluster, pilP, encodes a lipoprotein which is predicted to be implicated in stabilization of PilQ multimers (11). ORFs encoding PilP homologues are missing in the genome sequence of T. thermophilus HB27.
Characterization and possible functions of the identified competence genes.
The deduced protein of the first gene in this Thermus cluster is similar to PilM in P. aeruginosa and N. gonorrhoeae (Table 1). The PilM proteins in P. aeruginosa, N. gonorrhoeae, and T. thermophilus HB27 share a highly conserved C-terminal domain characteristic for FtsA cell division proteins. The presence of a total of three hydrophobic domains distributed over the whole Thermus PilM suggests that PilM is a cytoplasmic membrane protein. No clues to the function of PilN and PilO in Thermus can be derived from their homologues in P. aeruginosa and N. gonorrhoeae since their function in type IV pilus biogenesis is still unknown.
PilQ is similar to members of the secretin family, such as PilQ in Myxococcus xanthus (54), ExeD in Aeromonas salmonicida (24), PilQ in P. aeruginosa (31), and PilQ in N. gonorrhoeae (10). Secretins are conserved within a 250-amino acid (aa) C-terminal stretch, whereas the N-terminal and central parts are variable. As shown by Guilvout et al. (18), the C terminus of the Klebsiella secretin PulD is required for multimer formation, and it is concluded from their experiments that the β-domain of the C-terminal part is the major determinant of multimer stability. For the gonococcal PilQ, a dependence of multimerization on conserved C-terminal residues was also demonstrated (10). Since the C terminus in Thermus PilQ is well conserved, we conclude that the Thermus PilQ also forms ring-like structures which mediate DNA transport into the periplasm.
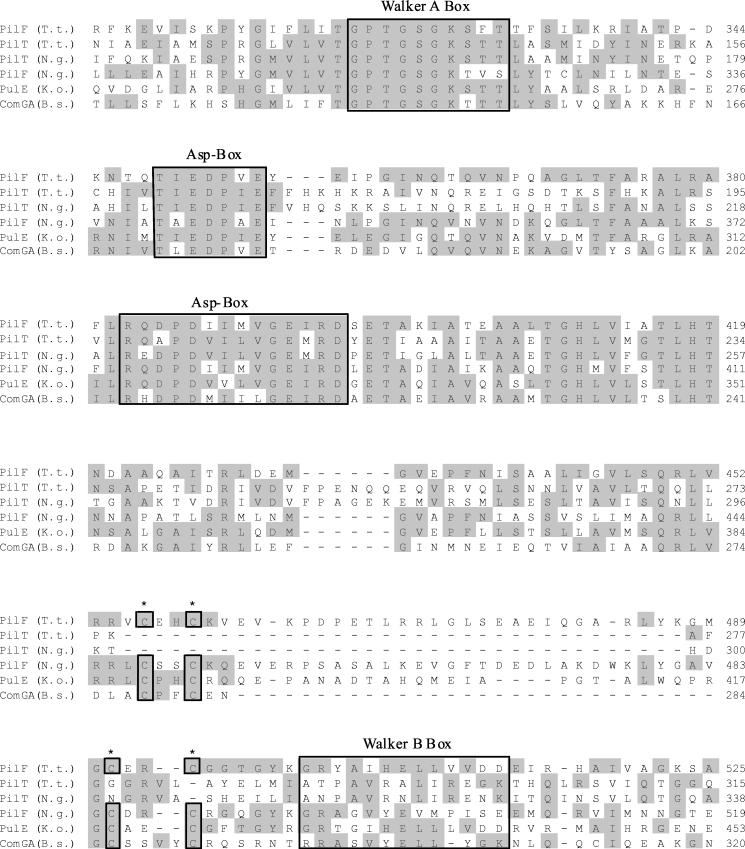
PilF (orf332) and PilT (orf333) are similar to pilus assembly proteins and to proteins of the general secretion pathway (Table 1). pilF (orf332) and pilT (orf333) are tightly clustered and analogously orientated (Fig. 1A). Both proteins contain a Walker A motif (53) and a conserved aspartate box (Fig. 4), and they are very similar to each other (49% similarity). Both motifs, the Walker A motif and the aspartate box (Fig. 4), are highly conserved in proteins of the PilT family (43). The two short motifs (TXEDPXE and RXXPDXXXGEI/MRD), containing at least one aspartate residue and therefore referred to as aspartate boxes, are typical for PulE-PilF-PilB and PilT homologues and are not found in other proteins with ATP-binding sites, such as ABC transporters (23, 43). The Walker A motif GXXXXGK(S/T)T of the Thermus PilF contains a phenylalanine in place of the second conserved threonine (Fig. 4). It has to be noted that this threonine residue is generally less conserved in PulE homologues, such as PilB in P. aeruginosa and PilF in Neisseria, which contain a valine instead. It has been shown by Possot and Pugsley (43) that replacement of key amino acids within the Walker A box of PulE, such as exchange of the conserved last glycine residue by alanine or of the conserved lysine residue by arginine, abolishes protein secretion in K. oxytoca. Analogously, Turner et al. (52) demonstrated that the highly conserved glycine residues within the Walker A box of the Pseudomonas proteins XcpR and PilB are essential for protein secretion and type IV pilus biogenesis, respectively, in P. aeruginosa. Likewise, the replacement of aspartate residues within each of the two aspartate boxes in PulE led to reduction of secretion efficiency in K. oxytoca (43). Although PilF and PilT share conserved regions, such as the Walker A motif and the conserved aspartate boxes, they are different in size (Fig. 1). Furthermore, a tetracysteine motif found in PilF is missing in PilT. This tetracysteine motif present in the C terminus of PilF (Fig. 4) resembles a zinc-binding motif. Such tetracysteine motifs are generally present in members of the PulE-PilB-PilF subgroup and some kinases such as adenylate kinases, but they are absent in proteins of the PilT subgroup. Since it was demonstrated in Klebsiella that this Cys motif is required for proper PulE function (44), we propose that this motif is important for the function of the Thermus PilF.
FIG. 4.
Alignment of the conserved central and C-terminal part of PilT-like proteins. Identical residues are indicated by grey shadows. The conserved cysteine residues found in PulE homologues are marked by a star (∗) and are boxed. B.s., B. subtilis; K.o., K. oxytoca; N.g. N. gonorrhoeae; T.t., T. thermophilus HB27.
orf1388 is the third ORF in the Thermus genome whose deduced protein exhibits conserved motifs characteristic for PilT proteins (Table 1) but, in contrast to pilF and pilT, an implication of orf1388 in natural transformation can be excluded. This observation clearly demonstrates that, despite their similarities, conserved proteins in Thermus do not necessarily belong to the same functional system, and it highlights the fact that sequence similarities might not (always) allow conclusions with respect to function, even in cases of highly conserved proteins.
The Thermus competence factor PilC (Table 2), which is closely related to the P. aeruginosa PilC type IV pilus protein (Table 1), is comprised of extended hydrophobic domains spanning the central region from aa 210 to 270 and spanning the last 40 C-terminal amino acids. It has been proposed that PilC-like proteins are polytopic integral membrane proteins, probably located in the cytoplasmic membrane (35, 42). The exact function of PilC-like proteins remains to be established, but it has been proposed that the Pseudomonas PilC and the Klebsiella homologue, PulF, are required for specific interactions with other type IV pilus assembly proteins (26, 43) and might be essential for their optimal localization or stabilization (26).
The product of orf2072 (PilD) is similar to prepilin peptidases. It has at least eight hydrophobic domains, which indicates a localization in the inner membrane. The highest identities of the Thermus PilD and prepilin peptidases are present within the N and C termini of the proteins. The N-terminal domain includes two pairs of cysteines which are required for the leader peptidase and methyltransferase activities of the bifunctional enzyme PilD, as demonstrated in P. aeruginosa (49). This motif is highly conserved, with the exception of XspO in Xanthomonas campestris (22), in all PilD proteins (30), and it is required for the proper function of prepilin peptidase proteins (44). The significant similarities, together with the highly conserved cysteine cluster, lead to the conclusion that PilD in T. thermophilus HB27 represents a prepilin peptidase most likely involved in proteolytic processing and methylation of prepilin-like proteins. It should be mentioned that no other prepilin peptidase was detected in the Thermus genome. Therefore, PilD should be implicated in the processing of all prepilins and prepilin-like proteins of T. thermophilus HB27.
The Thermus DprA (orf858) is similar to Smf and DprA proteins, which are widely distributed in transformable and nontransformable prokaryotes. DprA plays an essential role in transformation of H. influenzae, H. pylori, and S. pneumoniae (1, 6, 25). For the competence proteins of this family, a function in DNA transport through the cytoplasmic membrane and/or in recombination is postulated. The Thermus DprA and its homologues in H. influenzae have 57% similarity within a central 205-aa overlap (positions 60 to 265). This central region contains highly conserved amino acid stretches [VGXSR, positions 100 to 105; TSGLALGID(X3)H, positions 127 to 139; VLGS(X5)YP, positions 152 to 163; PRRNR, positions 190 to 194; and SGSLITA, positions 212 to 218]. The high degree of conservation indicates a critical function of these amino acid stretches.
Distinctive features of the Thermus transformation system.
The results presented here suggest that the DNA translocating machinery of T. thermophilus HB27 is related to type IV pili. In analogy to N. gonorrhoeae and P. stutzeri (15, 16), but in contrast to Acinetobacter sp. strain BD413 (28), the pili, as visible in electron micrographs, either are involved in natural transformation or pili and transformation are closely linked systems. This is concluded from the finding that the noncompetent mutants, with the exception of the pilF mutant, are devoid of pili structures. Despite the similarities of the components of the natural transformation machineries in Thermus and other transformable bacteria, the Thermus DNA translocation machinery is different from corresponding systems in other bacteria. The absence of a pilP homologue, the presence of a nonconserved pilW, and the low similarities of the Thermus PilN and PilO to homologues in other microorganisms might reflect the potential structural distinctiveness of the DNA transformation machinery in Thermus.
The suggestion that the Thermus transformation system differs from known transformation systems is also supported by the results obtained from characterization of the secretin-like PilQ protein in Thermus. Members of the secretin family form ring-like structures in the outer membrane which are implicated in type IV pilus biogenesis, protein export, and natural transformation of gram-negative bacteria, and also in phage assembly. It is suggested that the N terminus of secretins, which is implicated in protein export, folds back into the cavity of the channel that is formed by the C-terminal domain of the native complex. The N terminus of the secretin-like phage assembly protein pIV, which shows no homologies to the N termini of other secretins, is suggested to consist of a periplasmic substrate-binding domain that confers specificity to phage assembly (47). The N terminus of the Thermus PilQ shows no similarities to members of the secretin family, neither to secretins implicated in protein export or DNA import in gram-negative bacteria nor to proteins implicated in phage assembly. This finding indicates that the Thermus PilQ might interact with very distinct components.
The potential structural differences of the DNA transformation machinery in Thermus might be due to the structural distinctiveness of the cell envelope and the peptidoglycan of T. thermophilus. The murein from Thermus shows significant differences in complexity compared to the murein of other gram-negative bacteria; for example, the composition of murein and peptide cross bridges of T. thermophilus are typical for gram-positive bacteria, whereas the murein content, degree of cross-bridging, and glycan chain length are more similar to those from gram-negative bacteria. The outermost layer of the Thermus cell envelope is built by an S-layer covered by amorphous material (45). The distinct features of the Thermus cell envelope and the murein layer might have triggered the evolution of the pilMNOWQ cluster in Thermus.
Acknowledgments
This work was supported by grant Av 9/4-4 from the Deutsche Forschungsgemeinschaft. A. Friedrich was supported by the Stiftung Stipendien Fonds des Verbandes der Chemischen Industrie and the BMBF. The Göttingen Genomics Laboratory receives financial support from the Ministry of Science of the state of Lower Saxony.
We thank Caroline Wichmann and Olivia Gohl (Göttingen) for assistance with the electron microscopy studies.
REFERENCES
- 1.Ando, T., D. A. Israel, K. Kusugami, and M. J. Blaser. 1999. HP0333, a member of the dprA family, is involved in natural transformation in Helicobacter pylori. J. Bacteriol. 181:5572-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aravind, I., R. L. Tatusov, Y. I. Wolf, D. R. Walker, and E. V. Koonin. 1998. Evidence for massive gene exchange between archaeal and bacterial hyperthermophiles. Trends Genet. 14:442-444. [DOI] [PubMed] [Google Scholar]
- 3.Beckeral, H. D., B. Minal, C. Jacobib, G. Raczniaka, J. Pelaschiera, H. Royc, S. Kleinc, D. Kernc, and D. Sollad. 2000. The heterotrimeric Thermus thermophilus asp-tRNA (Asn) amidotransferase can also generate gln-tRNA. FEBS Lett. 476:140-144. [DOI] [PubMed] [Google Scholar]
- 4.Bhaya, D., N. R. Bianco, D. Bryant, and A. Grossman. 2000. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol. Microbiol. 37:941-9951. [DOI] [PubMed] [Google Scholar]
- 5.Brock, T. D. 1994. Genus Thermus Brock and Freeze 1969, 295AL, p. 333. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, Md. [Google Scholar]
- 6.Campbell, E. A., S. Y. Choi, and H. R. Masure. 1998. A competence regulon in Streptococcus pneumoniae revealed by genomic analysis. Mol. Microbiol. 27:929-939. [DOI] [PubMed] [Google Scholar]
- 7.de Grado, M., P. Castan, and J. Berenguer. 1999. A high-transformation-efficiency cloning vector for Thermus thermophilus. Plasmid 42:241-245. [DOI] [PubMed] [Google Scholar]
- 8.Doolittle, W. F. 1999. Lateral genomics. Trends Cell. Biol. 9:M5-M8. [PubMed] [Google Scholar]
- 9.Dougerthy, B. A., and H. O. Smith. 1999. Identification of Haemophilus infuenzae Rd transformation genes using cassette mutagenesis. Microbiology 145:401-409. [DOI] [PubMed] [Google Scholar]
- 10.Drake, S. L., and M. Koomey. 1995. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol. Microbiol. 18:975-986. [DOI] [PubMed] [Google Scholar]
- 11.Drake, S. L., S. A. Sandstedt, and M. Koomey. 1997. PilP, a pilus biogenesis lipoprotein in Neisseria gonorrhoeae, affects expression of PilQ as a high-molecular-mass multimer. Mol. Microbiol. 23:657-668. [DOI] [PubMed] [Google Scholar]
- 12.Dubnau, D. 1999. DNA uptake in bacteria. Annu. Rev. Microbiol. 53:217-244. [DOI] [PubMed] [Google Scholar]
- 13.Freitag, N. E., H. S. Seifert, and M. Koomey. 1995. Characterization of the pilF-pilD pilus assembly locus of Neisseria gonorrhoeae. Mol. Microbiol. 16:575-586. [DOI] [PubMed] [Google Scholar]
- 14.Friedrich, A., T. Hartsch, and B. Averhoff. 2001. Natural transformation in mesophilic and thermophilic bacteria: identification and characterization of novel, closely related competence genes in Acinetobacter sp. strain BD413 and Thermus thermophilus HB27. Appl. Environ. Microbiol. 67:3140-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fussenegger, M., T. Rudel, R. Barten, R. Ryll, and T. F. Meyer. 1997. Transformation competence and type IV pilus biogenesis in Neisseria gonorrhoeae. Gene 192:125-134. [DOI] [PubMed] [Google Scholar]
- 16.Graupner, S., V. Frey, R. Hashemi, M. G. Lorenz, G. Brandes, and W. Wackernagel. 2000. Type IV pilus genes pilA and pilC of Pseudomonas stutzeri are required for natural genetic transformation, and pilA can be replaced by corresponding genes from nontransformable species. J. Bacteriol. 182:2184-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graupner, S., and W. Wackernagel. 2001. Identification and characterization of novel competence genes comA and exbB involved in natural genetic transformation of Pseudomonas stutzeri. Res. Microbiol. 152:451-460. [DOI] [PubMed] [Google Scholar]
- 18.Guilvout, I., K. R. Hardie, N. Sauvonnet, and A. P. Pugsley. 1999. Genetic dissection of the outer membrane secretin PulD: are there distinct domains for multimerization and secretion specificity? J. Bacteriol. 181:7212-7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gwinn, M. L., R. Ramanathan, H. O. Smith, and J. F. Tomb. 1998. A new transformation-deficient mutant of Haemophilus influenzae Rd with normal DNA uptake. J. Bacteriol. 180:746-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hobbs, M., and J. S. Mattick. 1993. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol. Microbiol. 10:233-243. [DOI] [PubMed] [Google Scholar]
- 21.Hofreuter, D., S. Obenbreit, G. Henke, and R. Haas. 1998. Natural competence for DNA transformation in Helicobacter pylori: identification and genetic characterization of the comB locus. Mol. Microbiol. 28:1027-1038. [DOI] [PubMed] [Google Scholar]
- 22.Hu, N. T., P. F. Lee, and C. Chen. 1995. The type IV pre-pilin leader peptidase of Xanthomonas campestris pv. campestris is functional without conserved cysteine residues. Mol. Microbiol. 18:769-777. [DOI] [PubMed] [Google Scholar]
- 23.Hyde, S. C., P. Emsley, M. J. Hartshorn, M. M. Mimmack, U. Gileadi, S. R. Pearce, M. J. Gallagher, D. R. Gill, R. E. Hubbard, and C. F. Higgins. 1990. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature 346:362-365. [DOI] [PubMed] [Google Scholar]
- 24.Karlyshev, A. V., and S. MacIntyre. 1995. Cloning and study of the genetic organization of the exe gene cluster of Aeromonas salmonicida. Gene 158:77-82. [DOI] [PubMed] [Google Scholar]
- 25.Karudapuram, S., X. Zhao, and G. J. Barcak. 1995. DNA sequence and characterization of Haemophilus influenzae dprA+, a gene required for chromosomal but not plasmid DNA transformation. J. Bacteriol. 177:3235-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koga, T., K. Ishimoto, and S. Lory. 1993. Genetic and functional characterization of the gene cluster specifying expression of Pseudomonas aeruginosa pili. Infect. Immun. 61:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyama, Y., T. Hoshino, N. Tomizuka, and K. Furukawa. 1986. Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp. J. Bacteriol. 166:338-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Link, C., S. Eickernjäger, D. Porstendörfer, and B. Averhoff. 1998. Identification and characterization of a novel competence gene, comC, required for DNA binding and uptake in Acinetobacter sp. strain BD413. J. Bacteriol. 180:1592-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Londoño-Vallejo, J. A., and D. Dubnau. 1993. comF, a Bacillus subtilis competence locus, encodes a protein similar to ATP-dependent RNA/DNA helicases. Mol. Microbiol. 9:119-131. [DOI] [PubMed] [Google Scholar]
- 30.Lory, S., and M. S. Strom. 1997. Structure-function relationship of type IV prepilin peptidases of Pseudomonas aeruginosa. Gene 192:117-121. [DOI] [PubMed] [Google Scholar]
- 31.Martin, P. R., M. Hobbs, P. D. Free, Y. Jeske, and J. S. Mattick. 1993. Characterization of pilQ, a new gene required for the biogenesis of type 4 fimbriae in Pseudomonas aeruginosa. Mol. Microbiol. 9:857-868. [DOI] [PubMed] [Google Scholar]
- 32.Martin, P. R., A. A. Watson, T. F. McCaul, and J. S. Mattick. 1995. Characterization of a five gene cluster required for the biogenesis of type 4 fimbriae in Pseudomonas aeruginosa. Mol. Microbiol. 16:497-508. [DOI] [PubMed] [Google Scholar]
- 33.Mattick, J. S., C. B. Whitchurch, and R. A. Alm. 1996. The molecular genetics of type IV fimbriae in Pseudomonas aeruginosa. Gene 179:147-155. [DOI] [PubMed] [Google Scholar]
- 34.Mohan, S., J. Aghion, N. Guillen, and David Dubnau. 1989. Molecular cloning and characterization of comC, a late competence gene in Bacillus subtilis. J. Bacteriol. 171:6043-6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunn, D., S. Bergman, and S. Lory. 1990. Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J. Bacteriol. 172:2911-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oshima, T., and K. Imahori. 1974. Description of Thermus thermophilus comb. nov., a nonsporulating thermophilic bacterium (Yoshida and Oshima) from a Japanese thermal spa. Int. J. Syst. Bacteriol. 24:102-112. [Google Scholar]
- 37.Parkhill, J., M. Achtman, K. D. James, S. D. Bentley, C. Churcher, S. R. Klee, G. Morelli, D. Basham, D. Brown, T. Chillingworth, R. M. Davies, P. Davis, K. Devlin, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, S. Leather, S. Moule, K. Mungall, M. A. Quail, M. A. Rajandream, K. M. Rutherford, M. Simmonds, J. Skelton, S. Whitehead, B. G. Spratt, and B. G. Barrell. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 38.Pearce, B. J., A. M. Naughton, E. A. Campbell, and H. R. Masure. 1994. The rec locus, a competence-induced operon in Streptococcus pneumoniae. J. Bacteriol. 177:86-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pestova, E. V., and D. A. Morrison. 1998. Isolation and characterization of three Streptococcus pneumoniae transformation-specific loci by use of a lacZ reporter insertion vector. J. Bacteriol. 180:2701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porstendörfer, D., U. Drotschmann, and B. Averhoff. 1997. A novel competence gene, comP, is essential for natural transformation of Acinetobacter sp. BD413. Appl. Environ. Microbiol. 63:4150-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porstendörfer, D., O. Gohl, F. Mayer, and B. Averhoff. 2000. ComP, a pilin-like protein essential for natural competence in Acinetobacter sp. strain BD413: regulation, modification, and cellular localization. J. Bacteriol. 182:3673-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Possot, O., C. d'Enfert, I. Reyss, and A. P. Pugsley. 1992. Pullulanase secretion in Escherichia coli K-12 requires a cytoplasmic protein and a putative polytopic cytoplasmic membrane protein. Mol. Microbiol. 6:95-105. [DOI] [PubMed] [Google Scholar]
- 43.Possot, O., and A. P. Pugsley. 1994. Molecular characterization of PulE, a protein required for pullulanase secretion. Mol. Microbiol. 12:287-299. [DOI] [PubMed] [Google Scholar]
- 44.Possot, O., and A. P. Pugsley. 1997. The conserved tetracysteine motif in the general secretory pathway component PulE is required for efficient pullulanase secretion. Gene 192:45-50. [DOI] [PubMed] [Google Scholar]
- 45.Quintela, J. C., E. Pittenauer, G. Allmaier, V. Aran, and M. A. de Pedro. 1995. Structure of peptidoglycan from Thermus thermophilus HB8. J. Bacteriol. 177:4947-4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ropp, P. A, and R. A. Nicholas. 1997. Cloning and characterization of the ponA gene encoding penicillin-binding protein 1 from Neisseria gonorrhoeae and Neisseria meningitidis. J. Bacteriol. 179:2783-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russel, M., and B. Kazmierczak. 1993. Analysis of the structure and subcellular location of filamentous phage pIV. J. Bacteriol. 175:3998-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smeets, L. C., J. J. E. Bijlsma, S. Y. Boomkens, C. M. J. E. Vandenbroucke-Grauls, and J. G. Kusters. 2000. comH, a novel gene essential for natural transformation of Helicobacter pylori. J. Bacteriol. 182:3948-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strom, M. S., P. Bergman, and S. Lory. 1993. Identification of active-site cysteines in the conserved domain of PilD, the bifunctional type IV pilin leader peptidase/N-methyltransferase of Pseudomonas aeruginosa. J. Biol. Chem. 268:15788-15794. [PubMed] [Google Scholar]
- 50.Tonjum, R., N. E. Freitag, E. Namork, and M. Koomey. 1995. Identification and characterization of pilG, a highly conserved pilus assembly gene in pathogenic Neisseria. Mol. Microbiol. 16:451-464. [DOI] [PubMed] [Google Scholar]
- 51.Tonjum, T., and M. Koomey. 1997. The pilus colonization factor of pathogenic neisserial species: organelle biogenesis and structure/function relationships. Gene 192:155-163. [DOI] [PubMed] [Google Scholar]
- 52.Turner, L. R., J. Cano-Lara, D. N. Nunn, and S. Lory. 1993. Mutations in the consensus ATP-binding sites of XcpR and PilB eliminate extracellular protein secretion and pilus biogenesis in Pseudomonas aeruginosa. J. Bacteriol. 175:4962-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walker, J. R., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the α and β subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wall, D., P. E. Kolenbrander, and D. Kaiser. 1999. The Myxococcus xanthus pilQ (sglA) gene encodes a secretin homolog required for type IV pilus biogenesis, social motility, and development. J. Bacteriol. 181:24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitchurch, C. B., M. Hobbs, S. P. Livingson, V. Krishnapillai, and J. S. Mattick. 1991. Characterisation of a Pseudomonas aeruginosa twitching motility gene and evidence for a specialised protein export system widespread in eubacteria. Gene 101:33-44. [DOI] [PubMed] [Google Scholar]
- 56.White, O., J. A. Eisen, J. F. Heidelberg, E. K. Hickey, J. D. Peterson, et al. 1999. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science 286:1571-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfgang, M., J. P. M. Van Putten, S. F. Hayes, and M. Koomey. 1999. The comP locus of Neisseria gonorrhoeae encodes a type IV prepilin that is dispensable for pilus biogenesis but essential for natural transformation. Mol. Microbiol. 31:1345-1357. [DOI] [PubMed] [Google Scholar]
- 58.Yoshihara S., X. X. Geng, S. Okamoto, K. Yura, T. Murata, M. Go, M. Ohmori, and M. Ikeuchi. 2001. Mutational analysis of genes involved in pilus structure, motility and transformation competence in the unicellular motile cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 42:63-73. [DOI] [PubMed] [Google Scholar]