Abstract
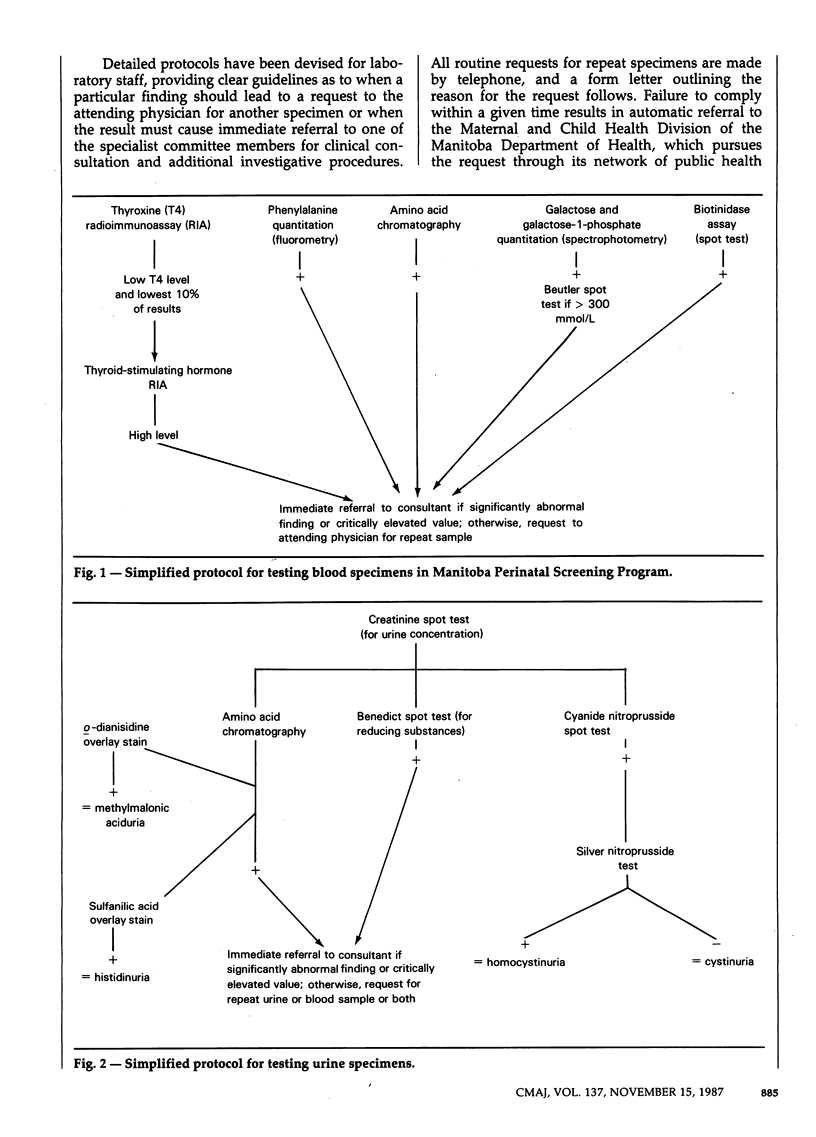
The Manitoba Perinatal Screening Program is guided by a committee of medical specialists with skills in the diagnosis and management of disorders of metabolism in the newborn. The program is voluntary and is centralized at Cadham Provincial Laboratory, in Winnipeg. A filter card blood specimen is collected from newborns on discharge from hospital, and a filter card urine sample is collected and mailed to the laboratory by the mother when the infant is about 2 weeks of age. The overall compliance rates for the blood and urine specimens are approximately 100% and 84% respectively. The blood specimen is screened for phenylalanine and other amino acids, thyroxine, galactose, galactose-1-phosphate and biotinidase. The urine specimen is screened for amino acids, including cystine, as well as methylmalonic acid and homocystine. Between 1965 and 1985, 83 cases of metabolic disorders were detected, including 23 cases of primary hypothyroidism, 14 of classic phenylketonuria, 5 of galactosemia variants, 3 of galactosemia, 2 of maple syrup urine disease and 1 of hereditary tyrosinemia. The direct cost per infant screened is $5.50, and the cost:benefit ratio is approximately 7.5:1. Maternal serum alpha-fetoprotein screening is being made available as the necessary supporting clinical facilities become available. On the basis of this experience, the author outlines the components that are important for an effective screening program.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doran T. A., Valentine G. H., Wong P. Y., Wielgosz G., Benzie R. J., Soltan H. C., Jenner M. R., Morland P. A., Montgomery R. J., Allen L. C. Maternal serum alpha-fetoprotein screening: report of a Canadian pilot project. CMAJ. 1987 Aug 15;137(4):285–293. [PMC free article] [PubMed] [Google Scholar]
- EFRON M. L., YOUNG D., MOSER H. W., MACCREADY R. A. A SIMPLE CHROMATOGRAPHIC SCREENING TEST FOR THE DETECTION OF DISORDERS OF AMINO ACID METABOLISM. A TECHNIC USING WHOLE BLOOD OR URINE COLLECTED ON FILTER PAPER. N Engl J Med. 1964 Jun 25;270:1378–1383. doi: 10.1056/NEJM196406252702602. [DOI] [PubMed] [Google Scholar]
- Fox J. G., Hall D. L., Haworth J. C., Maniar A., Sekla L. Newborn screening for hereditary metabolic disorders in Manitoba, 1965-1970. Can Med Assoc J. 1971 Jun 19;104(12):1085–1088. [PMC free article] [PubMed] [Google Scholar]
- Hofman L. F., Klaniecki J. E., Smith E. K. Direct solid-phase radioimmunoassay for screening 17 alpha-hydroxyprogesterone in whole-blood samples from newborns. Clin Chem. 1985 Jul;31(7):1127–1130. [PubMed] [Google Scholar]
- Wolf B., Grier R. E., Allen R. J., Goodman S. I., Kien C. L. Biotinidase deficiency: the enzymatic defect in late-onset multiple carboxylase deficiency. Clin Chim Acta. 1983 Jul 15;131(3):273–281. doi: 10.1016/0009-8981(83)90096-7. [DOI] [PubMed] [Google Scholar]
- Wolf B., Heard G. S., Jefferson L. G., Proud V. K., Nance W. E., Weissbecker K. A. Clinical findings in four children with biotinidase deficiency detected through a statewide neonatal screening program. N Engl J Med. 1985 Jul 4;313(1):16–19. doi: 10.1056/NEJM198507043130104. [DOI] [PubMed] [Google Scholar]


