Abstract
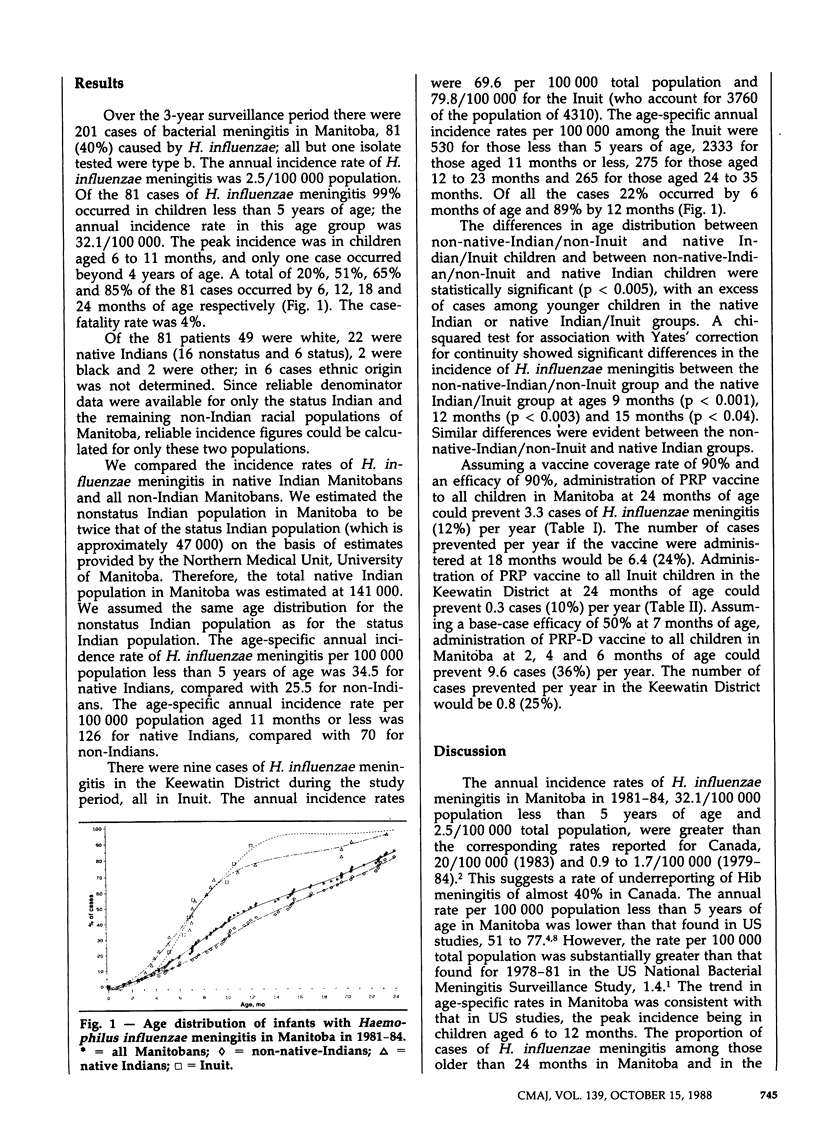
A community-based surveillance study of all central nervous system infections was carried out in Manitoba and the Keewatin District, NWT, between Apr. 1, 1981, and Mar. 31, 1984. There were 201 cases of bacterial meningitis in Manitoba over the study period, 81 (40%) caused by Haemophilus influenzae; all but one isolate tested were type b (Hib). There were nine cases of H. influenzae meningitis in the Keewatin District. The overall annual incidence rate of H. influenzae meningitis in Manitoba was 2.5/100,000; for children under 5 years the rate was 32.1/100,000. For the Keewatin District the corresponding rates were 69.6/100,000 and 530/100,000. A total of 85% and 100% of the cases of H. influenzae meningitis occurred by 24 months of age in Manitoba and the Keewatin District respectively. The age at onset was earlier in native Indian children (22 cases) and Inuit children (9 cases) than in non-native children (59 cases) (p less than 0.005); thus, vaccine prevention of Hib meningitis will likely be more difficult in native Indian and Métis children. Without evaluating the increased potential of H. influenzae vaccines to prevent nonmeningitic forms of disease, we concluded that mass childhood vaccination with polyribosylribitolphosphate (PRP) vaccine is not warranted in Manitoba or the Keewatin District. Immunogenicity studies suggest that administration of conjugated Hib vaccines such as PRP-D in infancy may prevent approximately one-third to two-thirds of cases of H. influenzae meningitis; these vaccines warrant consideration for use in mass childhood vaccination programs.
Full text
PDF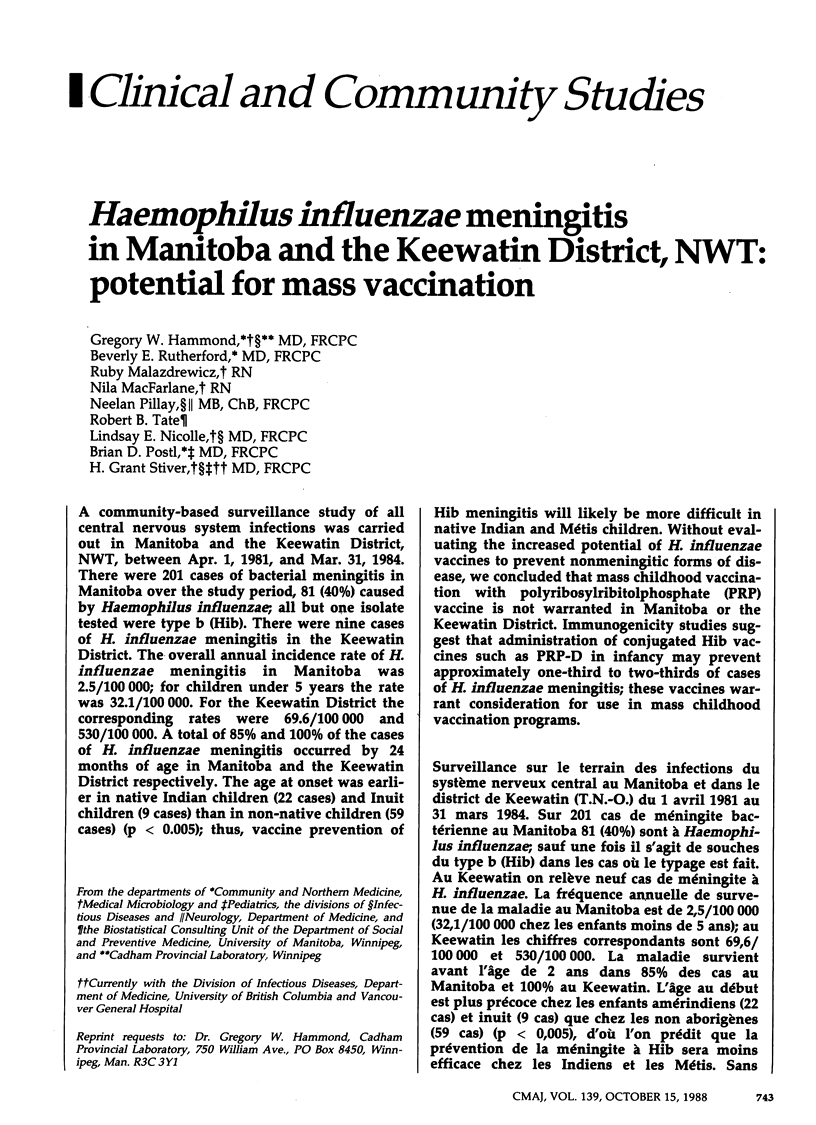



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cochi S. L., Broome C. V., Hightower A. W. Immunization of US children with Hemophilus influenzae type b polysaccharide vaccine. A cost-effectiveness model of strategy assessment. JAMA. 1985 Jan 25;253(4):521–529. [PubMed] [Google Scholar]
- Eskola J., Käyhty H., Peltola H., Karanko V., Mäkelä P. H., Samuelson J., Gordon L. K. Antibody levels achieved in infants by course of Haemophilus influenzae type B polysaccharide/diphtheria toxoid conjugate vaccine. Lancet. 1985 May 25;1(8439):1184–1186. doi: 10.1016/s0140-6736(85)92863-6. [DOI] [PubMed] [Google Scholar]
- Eskola J., Peltola H., Takala A. K., Käyhty H., Hakulinen M., Karanko V., Kela E., Rekola P., Rönnberg P. R., Samuelson J. S. Efficacy of Haemophilus influenzae type b polysaccharide-diphtheria toxoid conjugate vaccine in infancy. N Engl J Med. 1987 Sep 17;317(12):717–722. doi: 10.1056/NEJM198709173171201. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Koehler R. E., Fraser D. W. Race-specific differences in bacterial meningitis deaths in the United States, 1962-1968. Am J Public Health. 1976 Apr;66(4):392–396. doi: 10.2105/ajph.66.4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff D. M., Munson R. S., Jr Prospects for prevention of Haemophilus influenzae type b disease by immunization. J Infect Dis. 1986 Mar;153(3):448–461. doi: 10.1093/infdis/153.3.448. [DOI] [PubMed] [Google Scholar]
- Granoff D. M., Shackelford P. G., Suarez B. K., Nahm M. H., Cates K. L., Murphy T. V., Karasic R., Osterholm M. T., Pandey J. P., Daum R. S. Hemophilus influenzae type B disease in children vaccinated with type B polysaccharide vaccine. N Engl J Med. 1986 Dec 18;315(25):1584–1590. doi: 10.1056/NEJM198612183152505. [DOI] [PubMed] [Google Scholar]
- Hoban D. J., Witwicki E., Hammond G. W. Bacterial antigen detection in cerebrospinal fluid of patients with meningitis. Diagn Microbiol Infect Dis. 1985 Sep;3(5):373–379. doi: 10.1016/0732-8893(85)90075-6. [DOI] [PubMed] [Google Scholar]
- Käyhty H., Eskola J., Peltola H., Stout M. G., Samuelson J. S., Gordon L. K. Immunogenicity in infants of a vaccine composed of Haemophilus influenzae type b capsular polysaccharide mixed with DPT or conjugated to diphtheria toxoid. J Infect Dis. 1987 Jan;155(1):100–106. doi: 10.1093/infdis/155.1.100. [DOI] [PubMed] [Google Scholar]
- Moxon E. R. Haemophilus influenzae vaccine. Pediatrics. 1986 Feb;77(2):258–260. [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Virtanen M., Mäkelä P. H. Prevention of Hemophilus influenzae type b bacteremic infections with the capsular polysaccharide vaccine. N Engl J Med. 1984 Jun 14;310(24):1561–1566. doi: 10.1056/NEJM198406143102404. [DOI] [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Ward J. I., Band J. D., Hightower A., Fraser D. W., Broome C. V. Bacterial meningitis in the United States, 1978 through 1981. The National Bacterial Meningitis Surveillance Study. JAMA. 1985 Mar 22;253(12):1749–1754. [PubMed] [Google Scholar]
- Ward J. I., Margolis H. S., Lum M. K., Fraser D. W., Bender T. R., Anderson P. Haemophilus influenzae disease in Alaskan Eskimos: characteristics of a population with an unusual incidence of invasive disease. Lancet. 1981 Jun 13;1(8233):1281–1285. doi: 10.1016/s0140-6736(81)92458-2. [DOI] [PubMed] [Google Scholar]
- Wotton K. A., Stiver H. G., Hildes J. A. Meningitis in the central Arctic: a 4-year experience. Can Med Assoc J. 1981 Apr 1;124(7):887–890. [PMC free article] [PubMed] [Google Scholar]


