Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEEVERS H., GOLDSCHMIDT E. P., KOFFLER H. The use of esters of biologically active weak acids in overcoming permeability difficulties. Arch Biochem Biophys. 1952 Jul;39(1):236–238. doi: 10.1016/0003-9861(52)90282-8. [DOI] [PubMed] [Google Scholar]
- CHEN Y. T., ISHERWOOD F. A., MAPSON L. W. Quantitative estimation of ascorbic acid and related substances in biological extracts by separation on a paper chromatogram. Biochem J. 1953 Dec;55(5):821–823. doi: 10.1042/bj0550821. [DOI] [PMC free article] [PubMed] [Google Scholar]
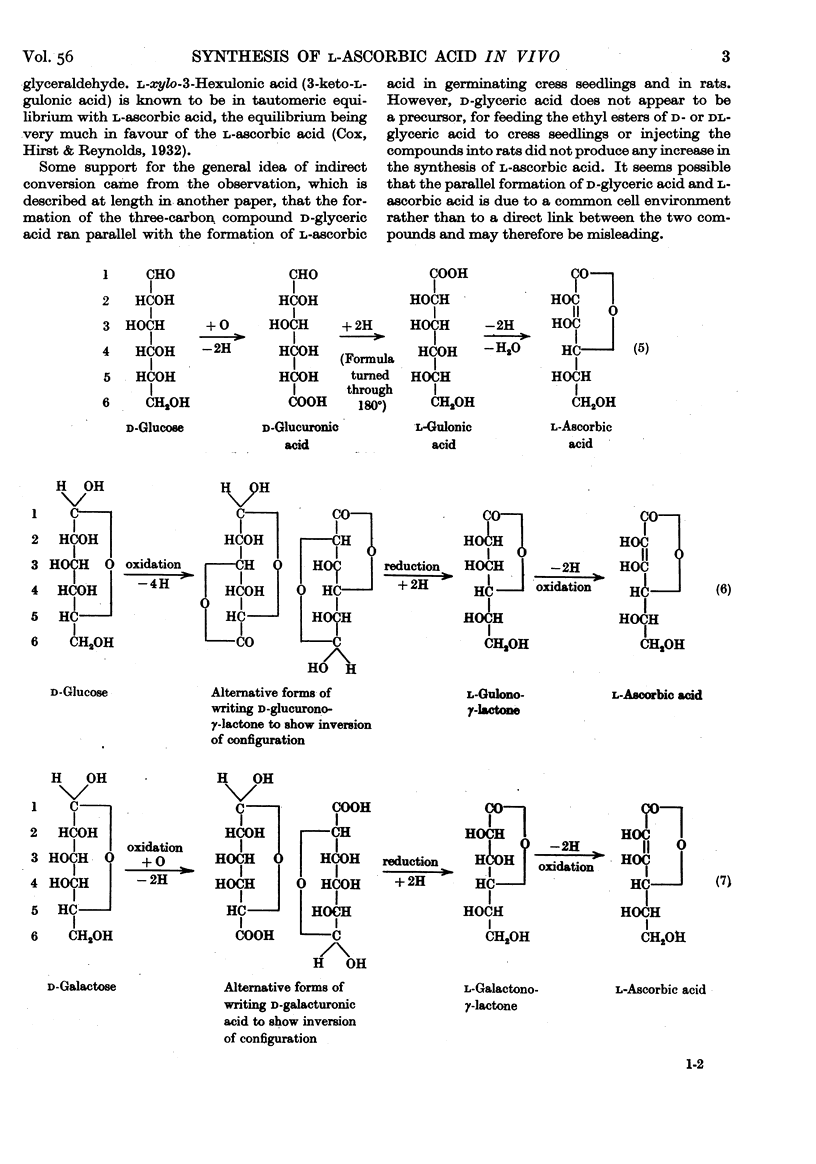
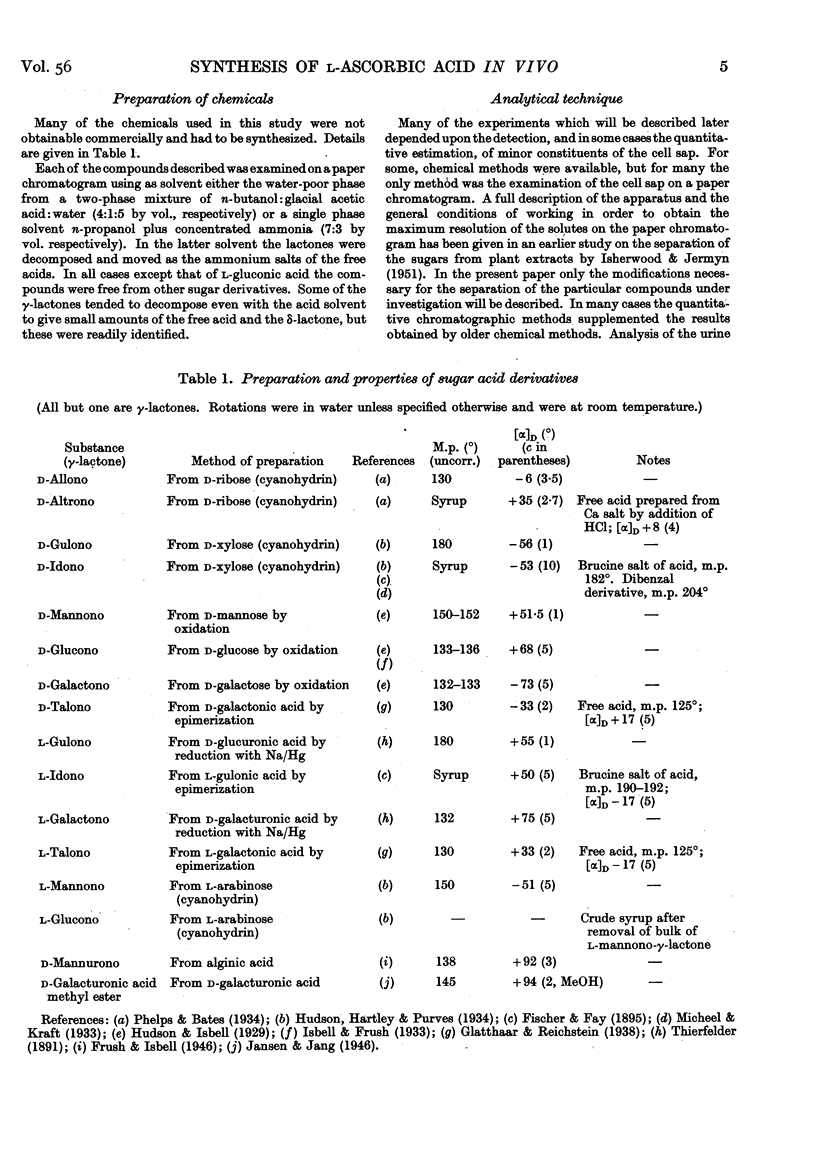
- HOROWITZ H. H., DOERSCHUK A. P., KING C. G. [The origin of L-ascorbic acid in the albino rat]. J Biol Chem. 1952 Nov;199(1):193–198. [PubMed] [Google Scholar]
- HOROWITZ H. H., KING C. G. The conversion of glucose-6-C14 to ascorbic acid by the albino rat. J Biol Chem. 1953 Jan;200(1):125–128. [PubMed] [Google Scholar]
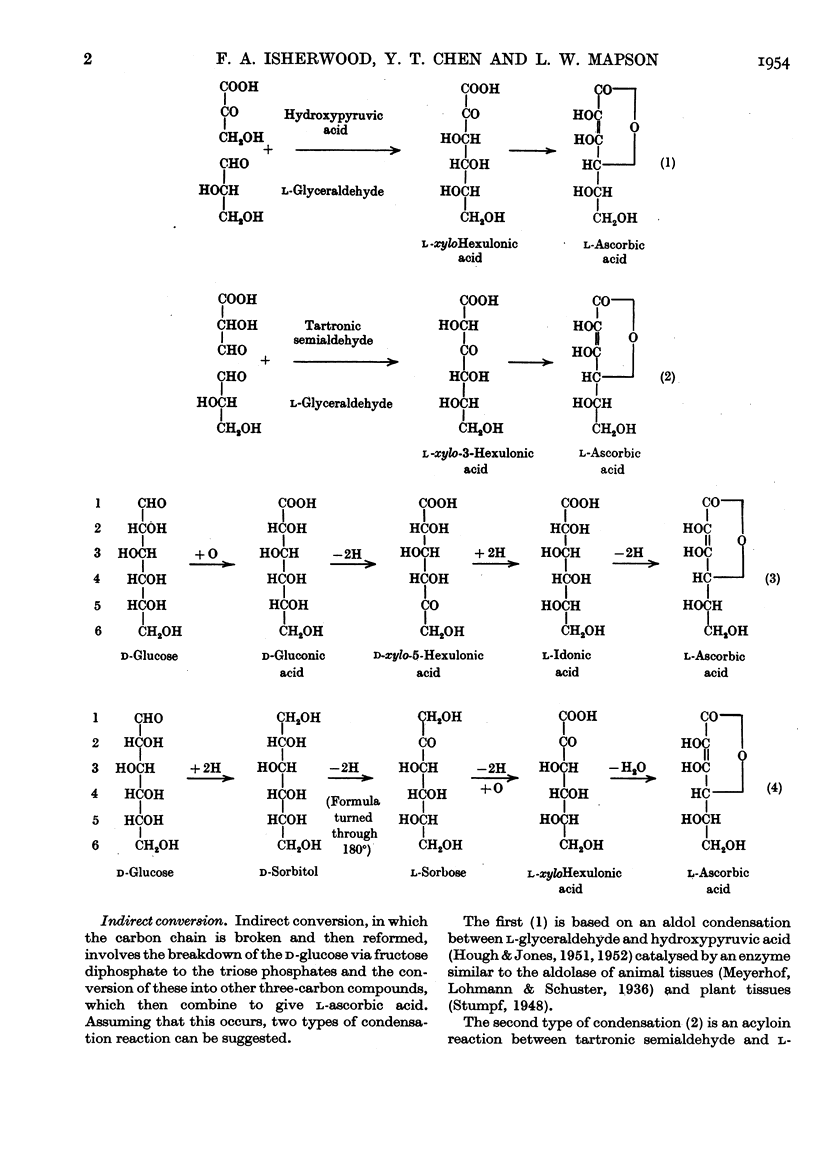
- HOUGH L., JONES J. K. N. Biosynthesis of the monosaccharides. Nature. 1951 Feb 3;167(4240):180–183. doi: 10.1038/167180a0. [DOI] [PubMed] [Google Scholar]
- Harris L. J., Olliver M. Vitamin methods: The reliability of the method for estimating vitamin C by titration against 2:6-dichlorophenolindophenol. 1. Control tests with plant tissues. Biochem J. 1942 Feb;36(1-2):155–182. doi: 10.1042/bj0360155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHERWOOD F. A., HANES C. S. Separation and estimation of organic acids on paper chromatograms. Biochem J. 1953 Dec;55(5):824–830. doi: 10.1042/bj0550824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHERWOOD F. A., JERMYN M. A. Relationship between the structure of the simple sugars and their behaviour on the paper chromatogram. Biochem J. 1951 May;48(5):515–524. doi: 10.1042/bj0480515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKEL S. S., MOSBACH E. H., BURNS J. J., KING C. G. The synthesis of l-ascorbic acid by the albino rat. J Biol Chem. 1950 Oct;186(2):569–579. [PubMed] [Google Scholar]
- Jermyn M. A., Isherwood F. A. Improved separation of sugars on the paper partition chromatogram. Biochem J. 1949;44(4):402–407. doi: 10.1042/bj0440402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARDY H. A., WIEBELHAUS V. D., MANN K. M. The mechanism by which glyceraldehyde inhibits glycolysis. J Biol Chem. 1950 Nov;187(1):325–337. [PubMed] [Google Scholar]
- LELOIR L. F. The enzymatic transformation of uridine diphosphate glucose into a galactose derivative. Arch Biochem Biophys. 1951 Sep;33(2):186–190. doi: 10.1016/0003-9861(51)90096-3. [DOI] [PubMed] [Google Scholar]
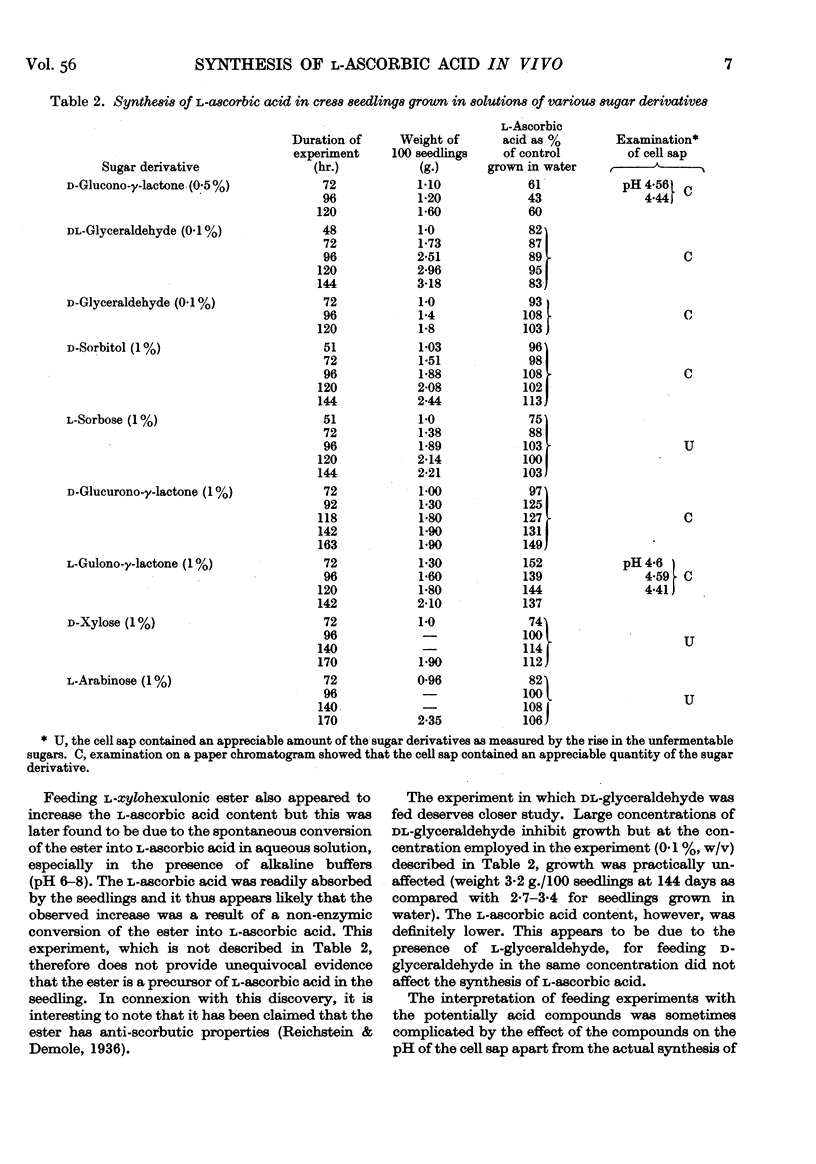
- Mapson L. W., Cruickshank E. M., Chen Y. T. Factors affecting synthesis of ascorbic acid in cress seedlings. 2. Ascorbic acid synthesis in relation to sugar formation. Biochem J. 1949;45(2):171–179. [PMC free article] [PubMed] [Google Scholar]
- Ray S. N. On the nature of the precursor of the vitamin C in the vegetable kingdom: Vitamin C in the growing pea seedling. Biochem J. 1934;28(3):996–1003. doi: 10.1042/bj0280996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON E. W., BEEVERS H. The quantitative relationship between pH and the activity of weak acids and bases in biological experiments. Science. 1951 Aug 3;114(2953):124–126. doi: 10.1126/science.114.2953.124. [DOI] [PubMed] [Google Scholar]


