Abstract
The etiology of cerebrovascular pathology is heterogeneous. Independent or synergistic role of this pathology relative to Alzheimer’s disease (AD) pathology is necessary to clarify distinct neurodegenerative pathways. We evaluated the interplay of various cerebrovascular markers postmortem and their in vivo neuroimaging, clinical and neuropathologic correlates using data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). In 109 individuals, postmortem cerebrovascular pathology (atherosclerosis of the circle of Willis, cerebral amyloid angiopathy [CAA], arteriolosclerosis, white matter rarefaction, old infarcts, microinfarcts, hemorrhages, other ischemic/vascular changes) was characterized. Additionally, we assessed in vivo neuroimaging (cortical thickness, subcortical volume, white matter lesion burden, glucose standardized uptake value ratio, fractional anisotropy of white matter tracts, cerebral blood flow), cognitive, and neuropathologic measures (atrophy, AD pathology and copathologies including Lewy body, TDP-43, hippocampal sclerosis). The study sample had mean (standard deviation) age of 82.9 (7.2) years and included 29 women (27%) and 84 (77%) with intermediate/high AD neuropathologic change. Arteriolosclerosis and CAA emerged as dominant cerebrovascular markers using multiple correspondence analysis. More severe arteriolosclerosis was explained by higher white matter lesion burden and greater postmortem hippocampal atrophy (β = 143.2, 95% CI 63.9 to 230.1, p = 0.0003), but not AD pathology. More severe CAA was explained by fractional anisotropy (β = − 20, 95% CI − 41.5 to -3.1, p = 0.02) adjusted for AD pathology and reduced integrity of superior cerebellar peduncle, posterior thalamic radiation, and sagittal stratum tracts (rho < − 0.6, false discovery rate corrected p < 0.05). More severe CAA was also explained by cortical atrophy and AD pathology (β = 0.6, 95% CI 0.2 to 1.2, p = 0.007), and associated with poorer memory (β = − 0.2, 95% CI − 0.3 to -0.09, p = 0.0009). Results demonstrate two dominant cerebrovascular pathways. An arteriolosclerosis-driven pathway is unspecific to AD pathology, whereas a CAA-driven pathway is specific to AD pathology. Cerebrovascular pathology is associated with AD pathology in an etiology-dependent manner which may influence eligibility for treatment or treatment-emergent adverse events in disease-modifying therapies for AD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00401-025-02970-8.
Keywords: Cerebrovascular pathology, Arteriolosclerosis, Cerebral amyloid angiopathy, Alzheimer’s disease, Copathology, Neurodegeneration
Introduction
Cerebrovascular disease is common in the aging brain, can cause stroke, drive vascular dementia, and is a common comorbid contributor to mixed dementias. Risk factors commonly associated with sporadic cerebrovascular disease include age and vascular factors such as hypertension and diabetes mellitus [30, 62]. Clinical consequences of cerebrovascular disease can be serious including stroke, progressive cognitive impairment, gait and posture disorders, and depression, leading to loss of independence in daily life [55].
Cerebrovascular disease encapsulates pathologies with heterogeneous etiology including vessel injury (e.g., arteriolosclerosis, atherosclerosis, cerebral amyloid angiopathy) and eventually irreversible tissue injury (e.g., infarcts, hemorrhages) [2, 38]. These injuries are detectable radiologically [17, 63] or histologically [51, 57]. There is not a single in vivo marker fully capturing the extent/type of this pathology. Instead, markers may be derived from a combination of sequences (structural, diffusion, arterial spin labeling) [17]. Given the different markers and varied etiology, the relationship between in vivo and postmortem markers of cerebrovascular pathology remains to be fully investigated.
Considering Alzheimer’s disease (AD) which is the most common cause of dementia, cerebrovascular disease has been reported as a copathology in 33–75% of the brains assessed postmortem [9, 35]. This is especially relevant for Aβ-lowering disease modifying immunotherapies in AD where cerebrovascular markers (e.g., microhemorrhages) can inform the risk of developing treatment-emergent adverse events such as amyloid-related imaging abnormalities (ARIA) [27]. Recognizing the contribution of comorbid pathologies [31], AD brains also commonly have Lewy body pathology [6], hippocampal sclerosis [10], and TAR DNA-binding protein 43 (TDP-43) [7]. Across both AD and non-AD dementias, cerebrovascular pathology has been shown to increase over age [1]. How these copathologies relate to cerebrovascular markers is yet another open question.
Thus, in this study, we investigated three questions in the context of AD: (1) Which are the dominant markers of cerebrovascular pathology? (2) Which are the in vivo, clinical, and neuropathologic correlates of the dominant cerebrovascular markers? (3) What are the possible mechanisms involving the dominant cerebrovascular markers in relation to neurodegeneration? We hypothesized that contribution of individual cerebrovascular markers would differ in relation to other cerebrovascular markers, AD markers, and neurodegeneration. Identifying key cerebrovascular markers and associated pathways could better inform the search for useful biomarkers for clinical trials, given the relevance of cerebrovascular pathology in recent disease-modifying trials for AD.
Materials and methods
Participants
We selected all available cases (110 individuals) from the Alzheimer’s Disease Neuroimaging Initiative (ADNI; RRID:SCR_003007) who underwent postmortem neuropathologic examination and included available in vivo neuroimaging and clinical data as of May 2024. The ADNI was launched in 2003 as a public–private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of the ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD as per www.adni-info.org. In general, individuals aged 55–90 years across cognitively normal, MCI and mild AD dementia groups were enrolled in the ADNI as per the inclusion criteria. Individuals with any significant neurologic disease other than AD were excluded. Of relevance to the current study, individuals showing evidence of infarction, focal lesions, or lacunes at baseline MRI were excluded. We selected individuals who consented to brain donation and underwent histopathologic assessment of postmortem biomarkers based on National Alzheimer's Coordinating Center (NACC) Neuropathology forms (version 10 and 11). Subsample of individuals had MRI (T1 weighted, diffusion tensor imaging, arterial spin labeling sequences) or FDG PET acquired at the time point closest to death which were also included. Inclusion/exclusion criteria and methodologies for neuropathologic, neuroimaging, and clinical data are documented and available at: https://adni.loni.usc.edu/help-faqs/adni-documentation/. All individuals provided written informed consent prior to participation in study procedures in accordance with the Declaration of Helsinki, and approval for the study was obtained by the local ethics committees of each participating site within ADNI. Procedures to obtain informed consent for postmortem assessment were followed as previously established [12]. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Image acquisition and processing
MRI
T1-weighted MRI were acquired on 1.5 T or 3 T scanners with sagittal 3D magnetization-prepared rapid gradient-echo sequences. MRI were pre-processed in-house [44] using cross-sectional FreeSurfer stream v6.0.0 (https://freesurfer.net/). Resulting segmentations were visually examined for quality control. Automatic region of interest parcellation yielded thickness of cortical structures and volumes of deep/subcortical structures [16, 20] measuring gray matter integrity. White matter hypointensity volume was used as an in vivo surrogate for cerebrovascular pathology [13, 41, 48]. All volume measures were normalized for estimated intracranial volume. Additionally, we examined AD signature thickness as surface-area weighted average of mean cortical thickness in the entorhinal, inferior temporal, middle temporal, and fusiform regions [32].
Diffusion tensor images (DTI) were acquired with 46 separate images including 5 T2-weighted images with no diffusion sensitization (b0 images) and 41 diffusion-weighted images (b = 1000 s/mm2). Processed DTI were obtained directly from ADNI which were screened for quality and pre-processed using FSL (www.fmrib.ox.ac.uk/fsl). Global and regional mean of fractional anisotropy in 40 bilateral white matter tracts of the JHU DTI-based white matter atlas [43] measured white matter microstructural integrity.
Arterial spin labeling (ASL) images were acquired with 3 T scanners and comprised 2D pulsed ASL (ADNI 2), 3D pulsed ASL, or 3D pseudo-continuous ASL (ADNI 3). Processed ASL were obtained directly from ADNI and included quality control, motion correction, perfusion-weighted image computation, geometric distortion and partial volume corrections, cerebral blood flow computation using a combination of FreeSurfer, FSL, SPM8 (http://www.fil.ion.ucl.ac.uk/spm), Insight Toolkit (ITK), and MATLAB. Global and regional mean cerebral blood flow was computed in the same brain regions as provided by FreeSurfer [16, 20] as a quantitative measure of blood flow in the capillary bed in the brain tissue.
FDG PET
FDG PET included dynamic 3D scans made up of six 5-min frames retrieved 30–60 min after administration of [18F] FDG. Processed FDG PET output was directly obtained from ADNI where coregistered and standardized images were spatially normalized to MNI PET template using SPM. Standardized uptake ratio (SUVR) scaled to pons/vermis as reference region was extracted for AD signature region (mean across bilateral angular gyrus, posterior cingulate, inferior temporal gyrus [39]). Additionally, SUVR was generated for regions provided by FreeSurfer [16, 20] using PETSurfer [25, 26].
Cognitive outcomes
We examined global cognition by Mini-Mental State Examination (MMSE) and cognitive domains by composite scores for memory, executive function, language, and visual–spatial abilities [45].
Postmortem neuropathology
Neuropathologic assessment of AD [42] was conducted following the NIA-AA guidelines within ADNI’s neuropathology core [22]. To analyze cerebrovascular pathology, we selected eight available markers: (1) atherosclerosis of the circle of Willis, (2) arteriolosclerosis in the subcortical white or gray matter, (3–6) old gross infarcts, old gross hemorrhages, old microinfarcts, or other pathologic changes related to ischemic or vascular disease (laminar necrosis, acute infarcts, vascular malformation, aneurysm, vasculitis, CADASIL, or mineralization of blood vessels) in the minimum recommended brain regions, (7) cerebral amyloid angiopathy (CAA) in the parenchymal, and/or leptomeningeal vessels in the minimum recommended brain regions, and (8) white matter rarefaction in the white matter pallor in the centrum semiovale and subcortical white matter. Minimum recommended brain regions [42] are medulla including dorsal motor nucleus of the vagus, pons including locus ceruleus, midbrain including substantia nigra, cerebellar cortex and dentate nucleus, thalamus and subthalamic nucleus, basal ganglia at the level of the anterior commissure with the basal nucleus of Meynert, hippocampus and entorhinal cortex, cingulate, anterior, amygdala, middle frontal gyrus, superior and middle temporal gyri, inferior parietal lobule, occipital cortex (Brodmann area 17 and 18), white matter at the anterior cerebral artery, middle cerebral artery, and posterior cerebral artery watershed. One participant was excluded due to missing values across multiple markers (three of eight including atherosclerosis of the circle of Willis, old gross infarcts including lacunes, old hemorrhages), leading to a final sample of 109 participants. We evaluated core AD pathologies by Thal stage, Braak stage, Consortium to Establish a Registry for Alzheimer's Disease (CERAD) score, and AD neuropathologic change; neurodegeneration by cortical and hippocampal atrophy; and non-AD copathologies by Lewy body pathology, TDP-43, hippocampal sclerosis. Neuropathologic variables were semi-quantitative and ordinal. Specifically, atherosclerosis, arteriolosclerosis, CAA, and white matter rarefaction were rated on a 4-point scale (none, mild, moderate, severe), while old gross infarcts, old gross hemorrhages, old microinfarcts, and other pathologic changes related to ischemic or vascular disease were binary and rated as either being present or absent.
Statistical analysis
To address our first question of examining the simultaneous relationship among the postmortem cerebrovascular markers to identify the dominant ones, we employed multiple correspondence analysis. This analysis offers an unsupervised data reduction approach for semi-qualitative variables [58]. Multiple correspondence analysis (RRID:SCR_014602) was used to model the eight cerebrovascular markers and simplify the model by minimizing the number of variables without a priori information about the correlation between variables to facilitate easier interpretation. Maximizing contribution of all variables, multiple correspondence analysis allows to model the variance from missing values after ruling out potential outliers. Missing data was treated as a separate factor level and treated as its own group in this model, which enables to understand how the missing group relates to other variables or categories in the multiple correspondence space without ignoring whole observations due to missing data only in a subset. The model is helpful in identifying a smaller number of uncorrelated axes describing the spread of data. To address our second question of identifying the correlates, we investigated the key axes identified by multiple correspondence analysis further by correlating them with neuroimaging markers (MRI, FDG PET, DTI, ASL) and cognitive (MMSE, cognitive domains) and neuropathologic variables (AD markers, atrophy, non-AD copathologies) using the Spearman’s partial correlation model [15] (correlation coefficient, rho). We reported p-values after controlling for false discovery rate (FDR), and pFDR < 0.05 was deemed significant. Based on the identified correlates, we addressed our third question of possible mechanisms related to the key axes identified by multiple correspondence analysis using regression analysis—ordinal logistic [28] and multiple linear regression for ordinal and continuous outcomes, respectively. In these conventional models computing partial correlations and regressions, missing data was handled by only including complete cases and dropping missing observations. The following covariates were included in the partial correlation and regression analyses: age at death if only postmortem data were included, difference in age (i.e., scan-to-postmortem examination interval) if both neuroimaging/cognitive and postmortem data were included, education for cognitive data, and relevant risk factors based on prior literature [8, 47, 53, 65] and availability of data (history of hypertension or APOE ɛ4 carriership). Analyses were performed in R v4.4.2 (RRID:SCR_000432).
Results
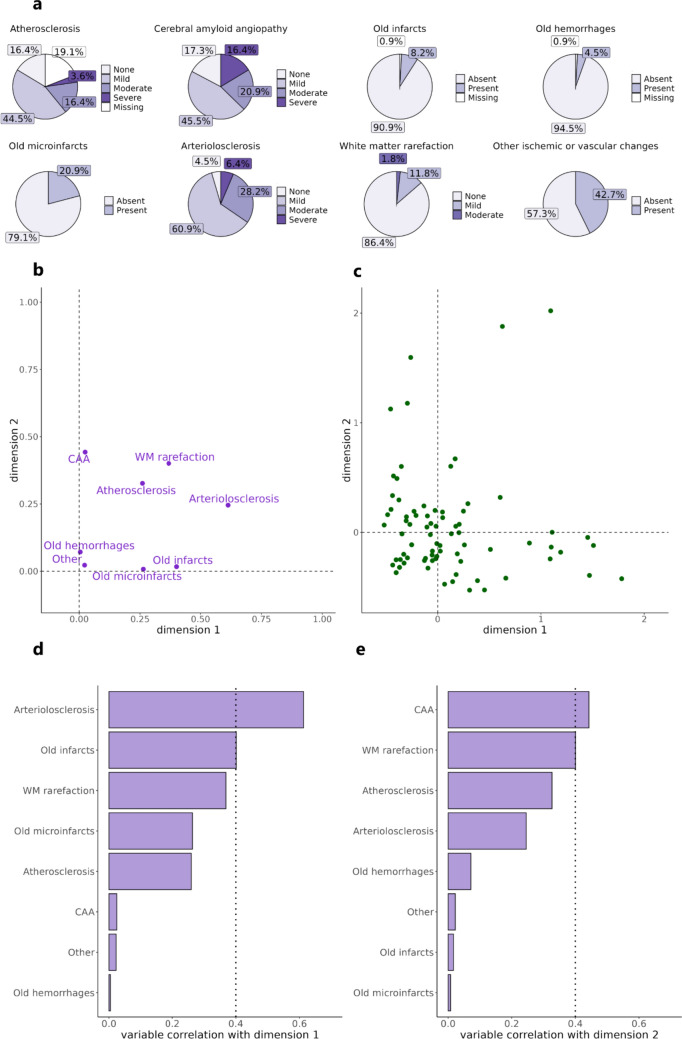
Demographics, clinical, and neuropathologic characteristics of study participants are presented in Table 1. The study sample was aged 83 ± 7 years at death, with 58% being APOE ɛ4 carriers, impaired in cognition (MMSE = 20 ± 7). Age difference (or interval) between neuroimaging-to-postmortem evaluations was systematically higher than that between neuroimaging-to-death. We note a high frequency of intermediate/high AD neuropathologic change postmortem (77%). Aside from the pathologies listed in Table 1, we did not stratify or adjust for other pathological subtypes due to missing data and/or limited variability (e.g., frontotemporal lobar degeneration with TDP-43 pathology was seen in two cases in the full sample, while frontotemporal lobar degeneration with tau pathology had 53% missing data and included two cases with progressive supranuclear palsy and none with corticobasal degeneration). Distribution of the eight cerebrovascular markers assessed postmortem is shown in Fig. 1a. All individuals showed the presence of at least one of these cerebrovascular markers. The majority (about ≥ 50%) showed some degree of atherosclerosis, CAA, arteriolosclerosis, or other vascular changes. The minority (about ≤ 20%) had evidence of white matter rarefaction, old gross infarcts, microinfarcts, and hemorrhages. Old cerebral microbleeds were absent in all participants and were, thus, excluded from analysis due to lack of variability. We evaluated ≥ 3 mm infarcts based on MRI (time from postmortem examination = 6.0 ± 3.5 years) detected by a trained expert directly available in ADNI. In this sample, 15 (13.8%) individuals showed MRI infarcts, localized in the thalamus (n = 5, one was hemorrhagic), basal ganglia (n = 3), cerebellum (n = 3), frontal cortex (n = 1), frontal white matter (n = 1), internal capsule (n = 1), and temporal white matter (n = 1, hemorrhagic). All others were thrombotic infarcts. About half of these cases (n = 7) showed the presence of one or more infarcts in neuropathologic examination (Supplementary Table 1).
Table 1.
Demographic, clinical, and neuropathologic characteristics of the study participants
| Variable | N | Value |
|---|---|---|
| Age at death (years) | 109 | 82.9 ± 7.2 |
| Sex (N, % women) | 109 | 29 (27%) |
| Education (years) | 109 | 16.2 ± 2.8 |
| Ethnicity | 109 | 96% White, 3% Black or African American, 1% Asian |
| Risk factors | ||
| APOE ɛ4 carriers (N, %) | 109 | 63 (58%) |
| History of hypertension (N, % present) | 109 | 63 (57%) |
| Cognition | ||
| Mini-Mental State Examination score | 109 | 19.9 ± 6.8 |
| Memory composite | 109 | − 0.9 ± 0.9 |
| Executive function composite | 109 | − 0.6 ± 0.9 |
| Language composite | 109 | − 0.5 ± 0.8 |
| Visuospatial ability composite | 109 | − 0.5 ± 0.7 |
| In vivo neuroimaging | ||
| MRI-to-death interval (years) | 109 | 3.2 ± 2.8 |
| FDG PET-to-death interval (years) | 88 | 3.9 ± 2.7 |
| DTI-to-death interval (years) | 20 | 2.6 ± 1.5 |
| ASL-to-death interval (years) | 14 | 3.1 ± 2.5 |
| MRI-to-postmortem examination interval (years) | 109 | 5.3 ± 3.5 |
| FDG PET-to-postmortem examination interval (years) | 88 | 5.7 ± 3.4 |
| DTI-to-postmortem examination interval (years) | 20 | 4.5 ± 1.9 |
| ASL-to-postmortem examination interval (years) | 14 | 5.1 ± 3.2 |
| Neuropathologic findings | ||
| Thal stage (N, % > Phase 2) | 109 | 96 (88%) |
| Braak stage (N, % > Stage II) | 109 | 86 (79%) |
| CERAD score (N, % > sparse) | 109 | 74 (68%) |
| AD neuropathologic change (N, % intermediate/high) | 109 | 84 (77%) |
| Lewy body (N, % present) | 109 | 54 (50%) |
| TDP-43 (N, % present) | 98 | 49 (55%) |
| Hippocampal sclerosis (N, % present) | 109 | 10 (9%) |
| Cortical atrophy (N, % present) | 92 | 83 (90%) |
| Hippocampal atrophy (N, % present) | 94 | 80 (85%) |
APOE ɛ4, ε4 allele of the apolipoprotein E, MRI magnetic resonance image, FDG PET fluorodeoxyglucose positron emission tomography, DTI diffusion tensor image, ASL arterial spin label, AD Alzheimer’s disease, CERAD Consortium to Establish a Registry for Alzheimer’s Disease, TDP-43 TAR DNA-binding protein 43 (TDP-43)
Fig. 1.
Distribution and relationship of the cerebrovascular markers. a Distribution of eight cerebrovascular markers in the study sample. b Two-dimensional representation obtained from multiple correspondence analysis which models the relationship among the eight cerebrovascular markers. c Distribution of individuals along the two dimensions obtained from multiple correspondence analysis. d Correlation of each cerebrovascular marker with dimension 1 and e dimension 2. Moderate correlation = 0.4 of each marker to the dimensions was considered as threshold for comparison (dotted line). CAA cerebral amyloid angiopathy, WM white matter
Arteriolosclerosis and CAA emerge as dominant among cerebrovascular markers
Firstly, we assessed the dominant cerebrovascular markers upon accounting for the interplay of the eight cerebrovascular markers using multiple correspondence analysis. Figure 1b-c shows the relationship of the eight cerebrovascular markers and distribution of participants along two dimensions. Two dimensions were chosen based on elbow method [4] (Supplementary Fig. 1) and showed a cumulative variance of 22%. We evaluated cerebrovascular markers showing moderate to strong correlation (> 0.4) with each dimension—arteriolosclerosis for dimension 1 (Fig. 1d; correlation = 0.61, p < 0.0001) and CAA for dimension 2 (Fig. 1e; correlation = 0.44, p < 0.0001).
Arteriolosclerosis and CAA have distinct correlates
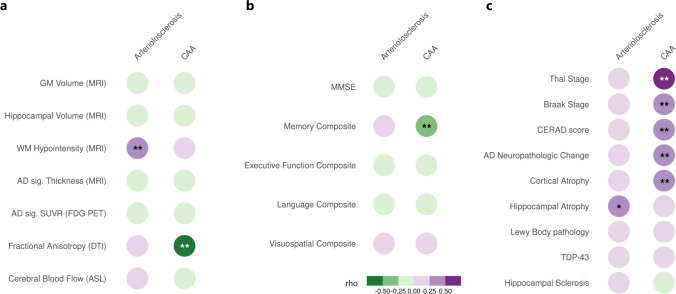
Secondly, we investigated in vivo, clinical and neuropathologic correlates of the dominant cerebrovascular markers using partial correlation models. Among in vivo neuroimaging correlates (Fig. 2a), arteriolosclerosis was associated with white matter hypointensity volume (rho = 0.30, pFDR = 0.03), while CAA was associated with mean fractional anisotropy (rho = − 0.55, pFDR = 0.049). We observed that neither total white matter volume (rho = − 0.06, p = 0.47) nor total white matter hypointensity volume (rho = 0.16, p = 0.08) was significantly associated with white matter rarefaction. Among cognitive correlates (Fig. 2b), CAA but not arteriolosclerosis was associated with memory composite score (rho = − 0.31, pFDR = 0.006). Among postmortem correlates (Fig. 2c), we only observed a marginally significant association between arteriolosclerosis and hippocampal atrophy (rho = 0.3, pFDR = 0.06). However, CAA was correlated with Thal stage (rho = 0.52, pFDR < 0.001), Braak stage (rho = 0.41, pFDR < 0.001), CERAD score (rho = 0.45, pFDR < 0.001), AD neuropathologic change (rho = 0.40, pFDR < 0.001), and cortical atrophy (rho = 0.25, pFDR = 0.049).
Fig. 2.
Correlates of arteriolosclerosis and cerebral amyloid angiopathy. Correlates of arteriolosclerosis and cerebral amyloid angiopathy with a in vivo neuroimaging measures based on MRI, FDG PET, DTI, ASL, b global and domain-specific cognitive scores (education-adjusted), and c postmortem AD and non-AD neuropathologic assessments. Partial correlations (rho) were visualized. Significant results indicated by ** correspond to pFDR < 0.05 and marginally significant result indicated by * corresponds to pFDR = 0.06. CAA cerebral amyloid angiopathy, MRI magnetic resonance imaging, GM gray matter, WM white matter, FDG PET [18F]fluorodeoxyglucose positron emission tomography, AD Alzheimer’s disease, AD sig. Alzheimer’s disease signature, DTI diffusion tensor imaging, ASL arterial spin labeling, MMSE Mini-Mental State Examination, CERAD Consortium to Establish a Registry for Alzheimer’s Disease, TDP-43 TAR DNA-binding protein 43 (TDP-43)
Differential association of arteriolosclerosis and CAA with neurodegeneration
Thirdly, we analyzed possible neurodegenerative mechanisms of cerebrovascular disease based on previous analyses. A significant two-way interaction in ordinal logistic regression indicated that individuals with higher in vivo white matter hypointensity volume and postmortem hippocampal atrophy showed more severe arteriolosclerosis (Fig. 3a; Supplementary Table 2: β = 143.2, 95% CI 63.9 to 230.1, p = 0.0003). Decreased global mean fractional anisotropy was significantly associated with more severe CAA seen in ordinal logistic regression (Supplementary Table 3: β = − 20, 95% CI − 41.5 to -3.1, p = 0.02). Further regional analyses using partial correlation showed that CAA severity was associated with decreased mean fractional anisotropy in three tracts (Fig. 3b) including superior cerebellar peduncle (rho = − 0.62, pFDR = 0.03), posterior thalamic radiation (rho = − 0.62, pFDR = 0.03), and sagittal stratum (rho = − 0.64, pFDR = 0.03). A significant two-way interaction in ordinal logistic regression indicated that individuals with intermediate/high ADNC and greater cortical atrophy postmortem showed more severe CAA (Fig. 3c; Supplementary Table 4: β = 0.6, 95% CI 0.2 to 1.2, p = 0.007). Further multiple linear regression showed that individuals with intermediate/high ADNC and more severe CAA exhibited poorer memory scores (Fig. 3d; Supplementary Table 5: β = − 0.2, 95% CI − 0.3 to − 0.09, p = 0.0009). Regional correlates of arteriolosclerosis and CAA based on MRI, FDG PET, and ASL did not survive multiple comparison corrections (Supplementary Fig. 2).
Fig. 3.
Possible mechanisms explaining the severity of arteriolosclerosis and cerebral amyloid angiopathy. a Severity of arteriolosclerosis was explained by a significant interaction of white matter hypointensity volume and hippocampal atrophy. White matter hypointensity volume is normalized by the estimated intracranial volume. Hippocampal atrophy was binarized as low (none or mild) or high (moderate or severe). The model was adjusted for difference in age between in vivo MRI and postmortem examination, and history of hypertension as a risk factor. b More severe CAA was associated with decreased fractional anisotropy of three white matter tracts including superior cerebellar peduncle, posterior thalamic radiation, and sagittal stratum. Fractional anisotropy values were averaged across hemispheres. Partial correlation for each tract is represented by rho for pFDR < 0.05. c Severity of cerebral amyloid angiopathy was explained by a significant interaction of cortical atrophy and ADNC status. The model was adjusted for age at death and APOE ɛ4 carriership as a risk factor. d Memory scores were explained by a significant interaction of cerebral amyloid angiopathy and ADNC status. The model was adjusted for difference in age between cognitive and postmortem examinations, education, and APOE ɛ4 carriership as a risk factor. Levels of arteriolosclerosis, CAA, and cortical atrophy include 1 = none, 2 = mild, 3 = moderate, and 4 = severe. CAA cerebral amyloid angiopathy, ADNC Alzheimer’s disease neuropathologic change
Discussion
Using a data-driven approach, we investigated the relationship among eight cerebrovascular markers postmortem. As hypothesized, some cerebrovascular markers are more closely linked to markers of AD than others. Two uncorrelated pathways emerged after accounting for inter-relationship among cerebrovascular markers with distinct imaging and clinical and pathologic correlates. One pathway was driven by arteriolosclerosis and associated with in vivo white matter lesion burden and postmortem hippocampal atrophy reflecting a non-AD-specific mechanism. A second pathway was driven by CAA and associated with poor in vivo white matter microstructural integrity, poor memory, postmortem cortical atrophy, and core AD hallmarks, reflecting an AD-specific mechanism. Interestingly, both arteriolosclerosis and CAA are major causes of small vessel disease [5, 30, 51] and represent vessel wall injury. Our findings added insights that parenchymal injury associated with each form of vessel wall injury is distinct.
Cerebrovascular pathology is increasingly being recognized as a driving or comorbid pathology in neurodegenerative diseases [31, 35]. There is ongoing debate on independent versus synergistic relationship of AD and cerebrovascular pathologies [3, 36, 37]. We observed that 77% of the sample had intermediate/high AD neuropathologic change (confirmed AD diagnosis), while incidence of cerebrovascular pathology ranged from 4.5 to 95.5% depending on the marker type. Heterogeneous manifestation of cerebrovascular pathology has been reported in vivo [17]7 and postmortem [57]9, making it challenging to disentangle its mechanisms from AD pathology. In a sample with limited cerebrovascular burden, we show here that the association between AD and cerebrovascular pathologies may be dependent on the etiology—arteriolosclerosis-related neurodegeneration did not vary by AD pathology, whereas CAA-related neurodegeneration was exacerbated for higher AD pathology.
Arteriolosclerosis is characterized by loss of smooth muscle cells, deposits of fibro-hyaline material, narrowing of the lumen, or vessel wall thickening of arterioles or arteries [51, 57]. Firstly, we found that more severe arteriolosclerosis was linked to higher in vivo white matter lesion burden. An explanation could be that arteriolosclerosis is most common in hypertensive individuals [29, 34] and white matter lesions can be induced due to hypertension [50]. Secondly, we found that more severe arteriolosclerosis was marginally associated with hippocampal atrophy postmortem. This may be possibly explained by the hippocampal sclerosis-aging pathogenesis hypothesis which posits a close link among systemic factors (e.g., hypertension), arteriolosclerosis, and hippocampal sclerosis [47]. Individuals with hippocampal sclerosis showed arteriolosclerosis in several cortical and subcortical regions, suggesting a brain-wide pathological process. It has been reported that hippocampal atrophy driven by hippocampal sclerosis, often overlapping with TDP-43 [46], is independent of AD pathology [49]. Third and importantly, we noted a significant interaction of in vivo white matter lesion burden and postmortem hippocampal atrophy explaining the severity of arteriolosclerosis. Interaction between white matter lesion burden and medial temporal atrophy has been reported in non-AD dementia [54]. Altogether, these findings may suggest that parenchymal injury associated with arteriolosclerosis follows a non-AD specific pathway—hippocampal atrophy may be partly attributed to Wallerian degeneration [18] from hypertension-induced white matter lesions and/or to non-AD pathologies such as hippocampal sclerosis.
CAA is characterized by progressive deposition of Aβ protein in the walls of arteries and arterioles in the leptomeningeal space, the cortex, and possibly in the capillaries and veins [51, 64]. Firstly, the severity of CAA was associated with decreased in vivo white matter microstructural integrity globally and regionally in three tracts including superior cerebellar peduncle, posterior thalamic radiation, and sagittal stratum. This finding is in line with prior evidence showing reduced integrity in the posterior tracts in Aβ + individuals with probable CAA compared to AD [33]. Stereotypical spread of CAA progresses from leptomeningeal and neocortical vessels to the medial temporal lobe and cerebellar vessels to the basal ganglia and thalamus [60]. White matter tracts showing compromised integrity in our findings align well with the advanced stages of CAA. A plausible mechanism could be that deposition of Aβ weakens vessel walls, disrupting arterioles which supply the deep white matter [40]. Secondly, CAA correlated with core AD hallmarks (Aβ, tau, CERAD, AD neuropathologic change). Of the cases with confirmed AD, 33% had advanced CAA (moderate–severe levels corresponding with substantial pathology in parenchymal and/or leptomeningeal vessels) within the estimated range of 25–44% for co-occurrence from prior studies [19, 65]. Despite shared involvement of Aβ in AD and CAA, they represent distinct entities [24]. Lastly, we observed a significant interaction such that advanced CAA and confirmed AD neuropathologic change explained poorer memory scores and postmortem cortical atrophy (Fig. 3d). This result suggests that co-occurring pathologies in AD can alter biological and clinical outcomes consistent with the revised criteria for AD [31], although caution should be exercised not to treat this result as a causal finding given the cross-sectional nature of our analysis. Regarding CAA-related cortical neurodegeneration, possible mechanisms may arise from concomitant parenchymal AD pathology, cortical effects of CAA-related structural lesions, or CAA-related vascular dysfunction [21]. Regarding CAA-related cognitive outcomes, it has been shown that interaction of advanced parenchymal Aβ in AD and vascular Aβ in CAA contribute to cognitive decline through tau pathology [52]. This interaction speaks to the synergistic interplay of cerebrovascular (CAA) and neurodegenerative (AD) pathologies in determining pathological and clinical deficits in AD. Taken together and in contrast to the mechanisms related to arteriosclerosis, parenchymal injury associated with CAA follows an AD-specific pathway.
Findings from this study have important implications in the context of Aβ-lowering disease modifying immunotherapies, as the mechanisms underlying the adverse events are closely related to brain vasculature, seen as ARIA [59]. It is currently recommended to exclude individuals with pre-existing cerebrovascular disease for treatment, particularly if there exist markers of CAA or ischemic brain injury [23]. However, risk factors for ARIA include APOE ε4 carriership and hypertension [11, 56, 61], which are key risk factors for CAA and arteriolosclerosis, respectively. The two pathways in this study may have differing implications for the approved treatments. CAA may help assess the risk of developing ARIA in individuals undergoing treatment, whereas arteriolosclerosis may help identify individuals at a higher risk of developing new infarcts, screening out individuals ineligible for treatment. Specific correlates of CAA (white matter microstructure and cortical atrophy adjusted for APOE ε4 carriership) and arteriolosclerosis (white matter lesion and hippocampal atrophy adjusted for hypertension) could be potential candidates for screening of individuals at risk for developing ARIA, thereby improving future clinical trial design. Such markers identified based on postmortem validation are necessary to develop reliable tools to maximize therapeutic benefits and minimize potential risks.
This study has some limitations. The overall sample size was modest, and subsamples based on DTI (n = 20) and ASL (n = 14) imaging were especially small, limiting the statistical power. Thus, the results based on these small samples should be treated with caution. However, there exist very few cohorts with both imaging sequences and postmortem data. While results should be reliable as they were evaluated against gold standard postmortem examination, further replication in independent cohorts is warranted. Some cerebrovascular markers were evaluated for presence or absence (especially markers of tissue injury such as infarcts, hemorrhages) and information about their severity would have been useful. Cerebral microbleeds constitute another cerebrovascular marker, which was absent postmortem in all individuals and due to lack of variability could not be assessed in this study. This may be explained by methodological considerations such as assessment of a limited number of sections of the cerebral cortex, subcortical white matter and periventricular white matter, subcortical gray matter, brainstem, and cerebellum. Microbleeds are closely related to CAA [14, 30] and should be investigated further. To identify dominant cerebrovascular markers, we considered two dimensions for ease of interpretation. Interaction of multiple cerebrovascular markers per dimension may be worth considering. Individuals with baseline evidence for infarctions, lacunes, and stroke or higher vascular risk were excluded in ADNI, which can underestimate cerebrovascular burden in the sample. Despite this limitation, we may still expect some cerebrovascular pathology to develop over time in older brains given that ADNI is a longitudinal study by design. However, the generalizability of the findings remains to be seen in general or clinical population where frequency of cerebrovascular pathology may differ.
In conclusion, this study identified distinct pathways within cerebrovascular pathology whose manifestation is heterogeneous. Arteriolosclerosis and cerebral amyloid angiopathy emerged as dominant markers of this pathology. Greater white matter lesion burden and hippocampal atrophy account for more severe arteriolosclerosis. Decreased white matter microstructural integrity, AD pathology, and cortical atrophy account for more severe cerebral amyloid angiopathy and poorer cognition. The pathway driven by arteriolosclerosis is rather unspecific to AD, while the one driven by cerebral amyloid angiopathy is AD specific. Thus, depending on the etiology, cerebrovascular pathology may act independently of or in tandem with AD pathology, leading to neurodegeneration.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Author contributions
Conceptualization: [Rosaleena Mohanty, Eric Westman]; Methodology: [Rosaleena Mohanty, Sophia Wheatley, Eric Westman]; Formal analysis and investigation: [Rosaleena Mohanty]; Writing—original draft preparation: [Rosaleena Mohanty]; Writing—review and editing: [Rosaleena Mohanty, Sophia Wheatley, Konstantinos Chiotis, Anna Marseglia, Eric Westman]; Funding acquisition: [Rosaleena Mohanty, Eric Westman].
Funding
Open access funding provided by Karolinska Institute. This study was funded by the Strategic Research Programme in Neuroscience at Karolinska Institutet (StratNeuro); the Swedish Research Council (VR) No. 2016–02282, 2021–01861, 2025–02405; the Center for Innovative Medicine (CIMED) No. FoUI-954459, FoUI-975174, FoUI-987392; the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet No. FoUI-952838, FoUI-954893; The Swedish Brain Foundation (Hjärnfonden) No. FO2022-0084, FO2024-0239; The Swedish Alzheimer's Foundation (Alzheimerfonden) No. AF-967495, AF-980387; Vinnova No. 2025–03749; The Swedish Parkinson's foundation (Parkinsonfonden) No. 1647/25, 1557/24, 1521/23; EU Innovative Health Initiative Joint Undertaking (IHI JU) AD-RIDDLE and ACCESS AD; King Gustaf V:s and Queen Victorias Foundation; Olle Engkvists Foundation (Olle Engkvists Stiftelse) No. 186–0660, 224–0069; the Åke Wiberg Foundation; Demensfonden; The Swedish Society for Medical Research (SSMF) PD21-0042; Neurofonden, Karolinska Institutet Research Grants, Foundation for Geriatric Diseases at Karolinska Institutet, Loo and Hans Osterman Foundation for Medical Research, The Lars Hierta Memorial Foundation, Gun och Bertil Stohne’s Foundation, The Foundation for Old Maids as well as Birgitta and Sten Westerberg for additional financial support. The funding sources did not have any involvement in the study design, collection, analysis, and interpretation of data, writing of the report; and the decision to submit the article for publication.
Data availability
This study used data from the Alzheimer's Disease Neuroimaging Initiative (ADNI). All ADNI data are shared through the LONI Image and Data Archive (IDA), a secure research data repository. Interested scientists may obtain access to ADNI imaging, clinical, genomic, and biomarker data for the purposes of scientific investigation, teaching, or planning clinical research studies. Access is contingent on adherence to the ADNI Data Use Agreement and the publication policies outlined in the documents listed below. The application process includes acceptance of the Data Use Agreement and submission of an online application form. The application must include the investigator’s institutional affiliation and the proposed uses of the ADNI. Link to the online repository: https://adni.loni.usc.edu/data-samples/adni-data/.
Declarations
Conflict of interest
None.
Footnotes
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (https://adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data, but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agrawal S, Schneider JA (2022) Vascular pathology and pathogenesis of cognitive impairment and dementia in older adults. Cereb Circ Cogn Behav 3:100148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA (2016) Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol 15(9):934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attems J, Jellinger KA (2014) The overlap between vascular disease and Alzheimer’s disease-lessons from pathology. BMC Med 12:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auerswald M, Moshagen M (2019) How to determine the number of factors to retain in exploratory factor analysis: a comparison of extraction methods under realistic conditions. Psychol Methods 24(4):468 [DOI] [PubMed] [Google Scholar]
- 5.Badji A, Youwakim J, Cooper A, Westman E, Marseglia A (2023) Vascular cognitive impairment–past, present, and future challenges. Ageing Res Rev 90:102042 [DOI] [PubMed] [Google Scholar]
- 6.Barba L, Abu-Rumeileh S, Barthel H, Massa F, Foschi M, Bellomo G et al (2024) Clinical and diagnostic implications of Alzheimer’s disease copathology in Lewy body disease. Brain 147(10):3325–3343 [DOI] [PubMed] [Google Scholar]
- 7.Besser LM, Teylan MA, Nelson PT (2020) Limbic predominant age-related TDP-43 encephalopathy (LATE): clinical and neuropathological associations. J Neuropathol Exp Neurol 79(3):305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blevins BL, Vinters HV, Love S, Wilcock DM, Grinberg LT, Schneider JA et al (2021) Brain arteriolosclerosis. Acta Neuropathol 141(1):1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA (2018) Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol 83(1):74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenowitz WD, Monsell SE, Schmitt FA, Kukull WA, Nelson PT (2014) Hippocampal sclerosis of aging is a key Alzheimer’s disease mimic: clinical-pathologic correlations and comparisons with both alzheimer’s disease and non-tauopathic frontotemporal lobar degeneration. J Alzheimers Dis 39(3):691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budd Haeberlein S, Aisen P, Barkhof F, Chalkias S, Chen T, Cohen S et al (2022) Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimer’s Dis 9(2):197–210 [DOI] [PubMed] [Google Scholar]
- 12.Cairns NJ, Taylor-Reinwald L, Morris JC, Initiative, A. s. D. N. (2010) Autopsy consent, brain collection, and standardized neuropathologic assessment of ADNI participants: the essential role of the neuropathology core. Alzheimers Dement 6(3):274–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cedres N, Ferreira D, Machado A, Shams S, Sacuiu S, Waern M et al (2020) Predicting Fazekas scores from automatic segmentations of white matter signal abnormalities. Aging Albany NY 12(1):894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charidimou A, Boulouis G, Frosch MP, Baron J-C, Pasi M, Albucher JF et al (2022) The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI–neuropathology diagnostic accuracy study. Lancet Neurol 21(8):714–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Winter JC, Gosling SD, Potter J (2016) Comparing the Pearson and Spearman correlation coefficients across distributions and sample sizes: a tutorial using simulations and empirical data. Psychol Methods 21(3):273 [DOI] [PubMed] [Google Scholar]
- 16.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31(3):968–980 [DOI] [PubMed] [Google Scholar]
- 17.Duering M, Biessels GJ, Brodtmann A, Chen C, Cordonnier C, de Leeuw F-E et al (2023) Neuroimaging standards for research into small vessel disease—advances since 2013. Lancet Neurol 22(7):602–618 [DOI] [PubMed] [Google Scholar]
- 18.Duering M, Righart R, Wollenweber FA, Zietemann V, Gesierich B, Dichgans M (2015) Acute infarcts cause focal thinning in remote cortex via degeneration of connecting fiber tracts. Neurology 84(16):1685–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis R, Olichney J, Thal L, Mirra S, Morris J, Beekly D et al (1996) Cerebral amyloid angiopathy in the brains of patients with Alzheimer’s disease: the CERAD experience, part XV. Neurology 46(6):1592–1596 [DOI] [PubMed] [Google Scholar]
- 20.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33(3):341–355 [DOI] [PubMed] [Google Scholar]
- 21.Fotiadis P, van Rooden S, van der Grond J, Schultz A, Martinez-Ramirez S, Auriel E et al (2016) Cortical atrophy in patients with cerebral amyloid angiopathy: a case-control study. Lancet Neurol 15(8):811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin EE, Perrin RJ, Vincent B, Baxter M, Morris JC, Cairns NJ et al (2015) Brain collection, standardized neuropathologic assessment, and comorbidity in Alzheimer’s Disease Neuroimaging Initiative 2 participants. Alzheimer’s Dement 11(7):815–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg SM, Aparicio HJ, Furie KL, Goyal MS, Hinman JD, Kozberg M et al (2025) Vascular neurology considerations for antiamyloid immunotherapy: a science advisory from the American Heart Association. Stroke 56(1):e30–e38 [DOI] [PubMed] [Google Scholar]
- 24.Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ (2020) Cerebral amyloid angiopathy and Alzheimer disease—one peptide, two pathways. Nat Rev Neurol 16(1):30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greve DN, Salat DH, Bowen SL, Izquierdo-Garcia D, Schultz AP, Catana C et al (2016) Different partial volume correction methods lead to different conclusions: an 18F-FDG-PET study of aging. Neuroimage 132:334–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greve DN, Svarer C, Fisher PM, Feng L, Hansen AE, Baare W et al (2014) Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. Neuroimage 92:225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hampel H, Elhage A, Cho M, Apostolova LG, Nicoll JA, Atri A (2023) Amyloid-related imaging abnormalities (ARIA): radiological, biological and clinical characteristics. Brain 146(11):4414–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrell FE Jr (2015) Ordinal logistic regression. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. Springer, New York, pp 311–325 [Google Scholar]
- 29.Ighodaro ET, Abner EL, Fardo DW, Lin A-L, Katsumata Y, Schmitt FA et al (2017) Risk factors and global cognitive status related to brain arteriolosclerosis in elderly individuals. J Cereb Blood Flow Metab 37(1):201–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue Y, Shue F, Bu G, Kanekiyo T (2023) Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease. Mol Neurodegener 18(1):46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jack CR Jr, Andrews JS, Beach TG, Buracchio T, Dunn B, Graf A et al (2024) Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimers Dement 20(8):5143–5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jack CR Jr, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS et al (2017) Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement 13(3):205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jo S, Cheong E-N, Kim N, Oh JS, Shim WH, Kim H-J et al (2022) Role of white matter abnormalities in the relationship between microbleed burden and cognitive impairment in cerebral amyloid angiopathy. J Alzheimers Dis 86(2):667–678 [DOI] [PubMed] [Google Scholar]
- 34.Kang C-K, Park C-A, Lee H, Kim S-H, Park C-W, Kim Y-B et al (2009) Hypertension correlates with lenticulostriate arteries visualized by 7T magnetic resonance angiography. Hypertension 54(5):1050–1056 [DOI] [PubMed] [Google Scholar]
- 35.Kapasi A, DeCarli C, Schneider JA (2017) Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 134:171–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HJ, Park S, Cho H, Jang YK, Lee J, Jang H et al (2018) Assessment of extent and role of tau in subcortical vascular cognitive impairment using 18F-AV1451 positron emission tomography imaging. JAMA Neurol 75(8):999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laing KK, Simoes S, Baena-Caldas GP, Lao PJ, Kothiya M, Igwe KC et al (2020) Cerebrovascular disease promotes tau pathology in Alzheimer’s disease. Brain Commun 2(2):fcaa132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamar M, Leurgans S, Kapasi A, Barnes LL, Boyle PA, Bennett DA et al (2022) Complex profiles of cerebrovascular disease pathologies in the aging brain and their relationship with cognitive decline. Stroke 53(1):218–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL et al (2011) Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 32(7):1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu M, Nie Z-Y, Li R-R, Zhang W, Wang H, He Y-S et al (2018) Correlation of brain perfusion with white matter hyperintensity, brain atrophy, and cognition in patients with posterior cerebral artery stenosis and subjective cognitive decline. Med Sci Monit 24:5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohanty R, Ferreira D, Westman E (2024) Multi-pathological contributions toward atrophy patterns in the Alzheimer’s disease continuum. Front Neurosci 18:1355695. 10.3389/fnins.2024.1355695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW et al (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K et al (2008) Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40(2):570–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muehlboeck J-S, Westman E, Simmons A (2014) TheHiveDB image data management and analysis framework. Front Neuroinform 7:49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukherjee S, Choi S-E, Lee ML, Scollard P, Trittschuh EH, Mez J et al (2023) Cognitive domain harmonization and cocalibration in studies of older adults. Neuropsychology 37(4):409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA et al (2015) Hippocampal sclerosis and TDP-43 pathology in aging and A lzheimer disease. Ann Neurol 77(6):942–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neltner JH, Abner EL, Baker S, Schmitt FA, Kryscio RJ, Jicha GA et al (2014) Arteriolosclerosis that affects multiple brain regions is linked to hippocampal sclerosis of ageing. Brain 137(1):255–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nemy M, Cedres N, Grothe MJ, Muehlboeck J-S, Lindberg O, Nedelska Z et al (2020) Cholinergic white matter pathways make a stronger contribution to attention and memory in normal aging than cerebrovascular health and nucleus basalis of Meynert. Neuroimage 211:116607 [DOI] [PubMed] [Google Scholar]
- 49.Ortega-Cruz D, Iglesias JE, Rabano A, Strange BA (2023) Hippocampal sclerosis of aging at post-mortem is evident on MRI more than a decade prior. Alzheimers Dement 19(11):5307–5315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ovbiagele B, Saver JL (2006) Cerebral white matter hyperintensities on MRI: current concepts and therapeutic implications. Cerebrovasc Dis 22(2–3):83–90 [DOI] [PubMed] [Google Scholar]
- 51.Pantoni L (2010) Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9(7):689–701 [DOI] [PubMed] [Google Scholar]
- 52.Rabin JS, Nichols E, La Joie R, Casaletto KB, Palta P, Dams-O’Connor K et al (2022) Cerebral amyloid angiopathy interacts with neuritic amyloid plaques to promote tau and cognitive decline. Brain 145(8):2823–2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rannikmäe K, Kalaria RN, Greenberg SM, Chui HC, Schmitt FA, Samarasekera N et al (2014) APOE associations with severe CAA-associated vasculopathic changes: collaborative meta-analysis. J Neurol Neurosurg Psychiatry 85(3):300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rennie A, Ekman U, Shams S, Rydén L, Samuelsson J, Zettergren A et al (2024) Cerebrovascular and Alzheimer’s disease biomarkers in dementia with Lewy bodies and other dementias. Brain Commun 6(5):fcae290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rensma SP, van Sloten TT, Launer LJ, Stehouwer CD (2018) Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci Biobehav Rev 90:164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J et al (2023) Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330(6):512–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skrobot OA, Attems J, Esiri M, Hortobágyi T, Ironside JW, Kalaria RN et al (2016) Vascular cognitive impairment neuropathology guidelines (VCING): the contribution of cerebrovascular pathology to cognitive impairment. Brain 139(11):2957–2969 [DOI] [PubMed] [Google Scholar]
- 58.Sourial N, Wolfson C, Zhu B, Quail J, Fletcher J, Karunananthan S et al (2010) Correspondence analysis is a useful tool to uncover the relationships among categorical variables. J Clin Epidemiol 63(6):638–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sperling RA, Jack CR Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT et al (2011) Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement 7(4):367–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thal DR, Ghebremedhin E, Orantes M, Wiestler OD (2003) Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J Neuropathol Exp Neurol 62(12):1287–1301 [DOI] [PubMed] [Google Scholar]
- 61.Van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M et al (2023) Lecanemab in early Alzheimer’s disease. N Engl J Med 388(1):9–21 [DOI] [PubMed] [Google Scholar]
- 62.van Norden AG, de Laat KF, Gons RA, van Uden IW, van Dijk EJ, van Oudheusden LJ et al (2011) Causes and consequences of cerebral small vessel disease. The RUN DMC study: a prospective cohort study. Study rationale and protocol. BMC Neurol 11:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R et al (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12(8):822–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamaguchi H, Yamazaki T, Lemere C, Frosch M, Selkoe D (1992) Beta amyloid is focally deposited within the outer basement membrane in the amyloid angiopathy of Alzheimer’s disease. An immunoelectron microscopic study. Am J Pathol 141(1):249 [PMC free article] [PubMed] [Google Scholar]
- 65.Yu L, Boyle PA, Nag S, Leurgans S, Buchman AS, Wilson RS et al (2015) APOE and cerebral amyloid angiopathy in community-dwelling older persons. Neurobiol Aging 36(11):2946–2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study used data from the Alzheimer's Disease Neuroimaging Initiative (ADNI). All ADNI data are shared through the LONI Image and Data Archive (IDA), a secure research data repository. Interested scientists may obtain access to ADNI imaging, clinical, genomic, and biomarker data for the purposes of scientific investigation, teaching, or planning clinical research studies. Access is contingent on adherence to the ADNI Data Use Agreement and the publication policies outlined in the documents listed below. The application process includes acceptance of the Data Use Agreement and submission of an online application form. The application must include the investigator’s institutional affiliation and the proposed uses of the ADNI. Link to the online repository: https://adni.loni.usc.edu/data-samples/adni-data/.