Abstract
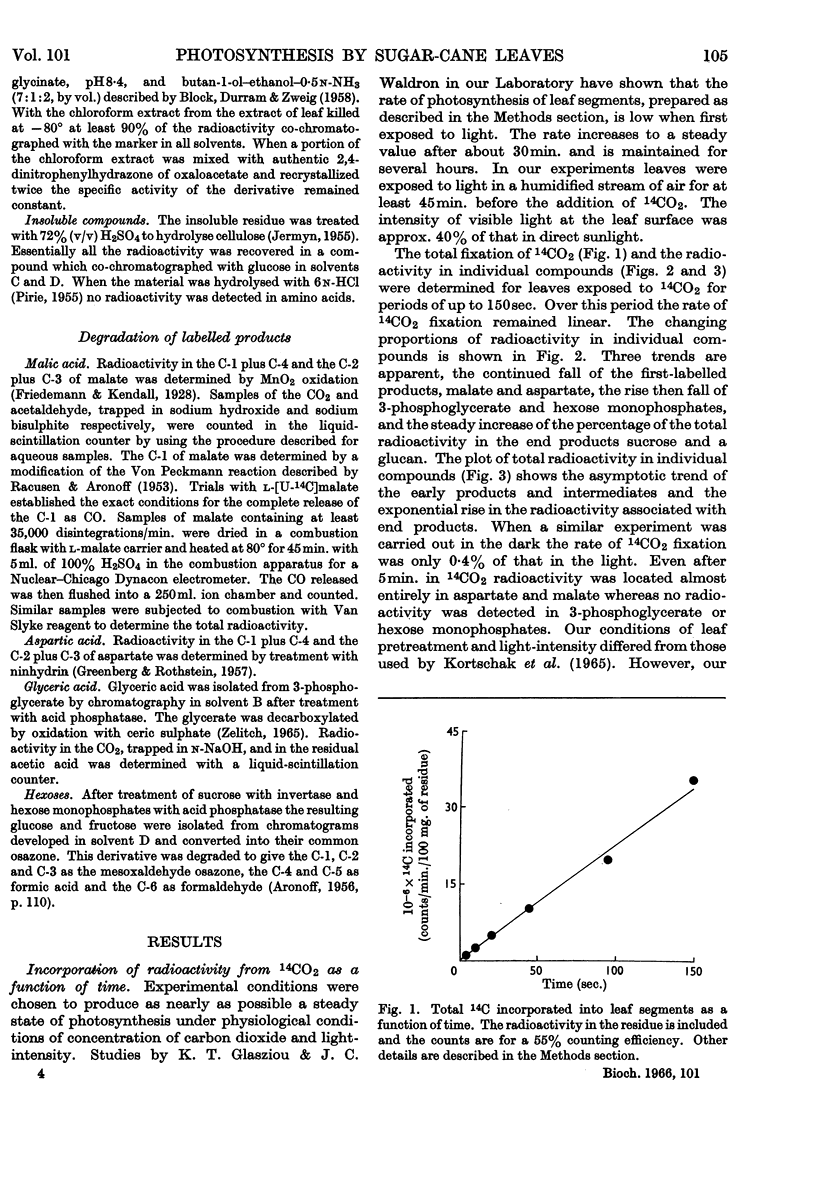
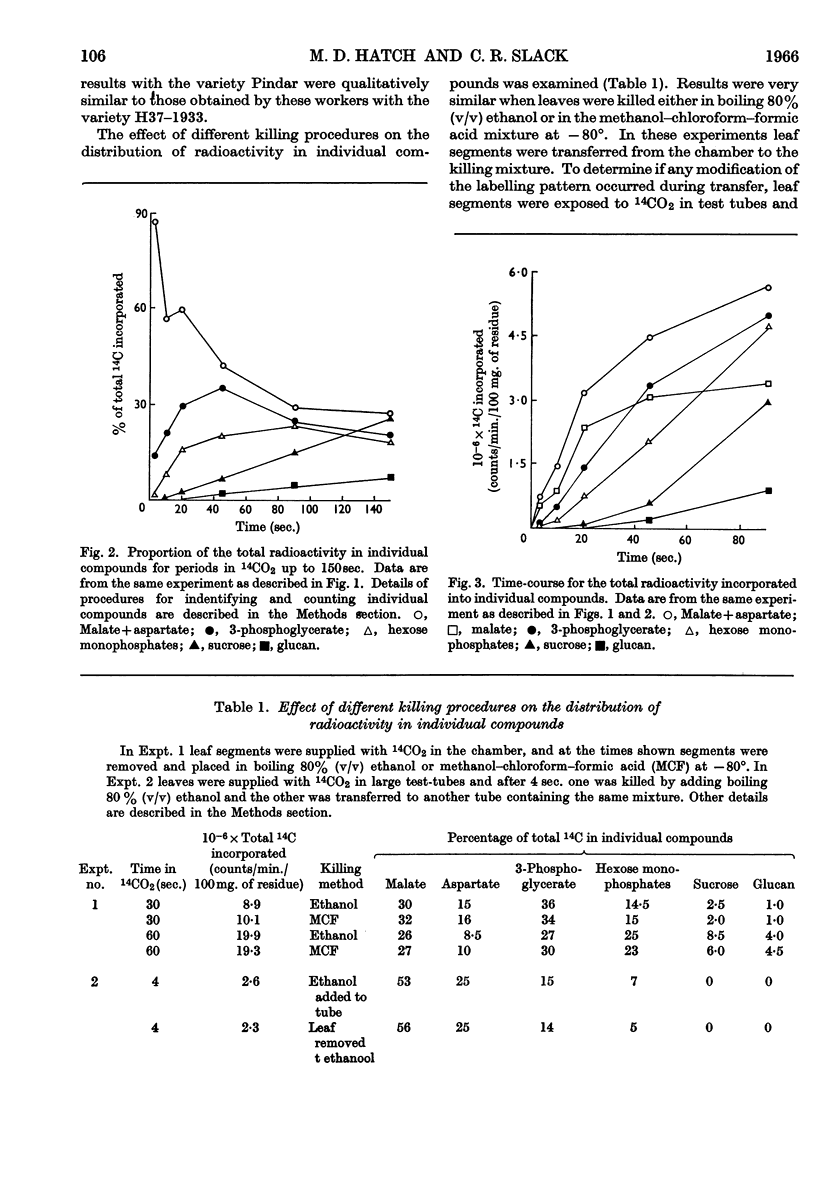
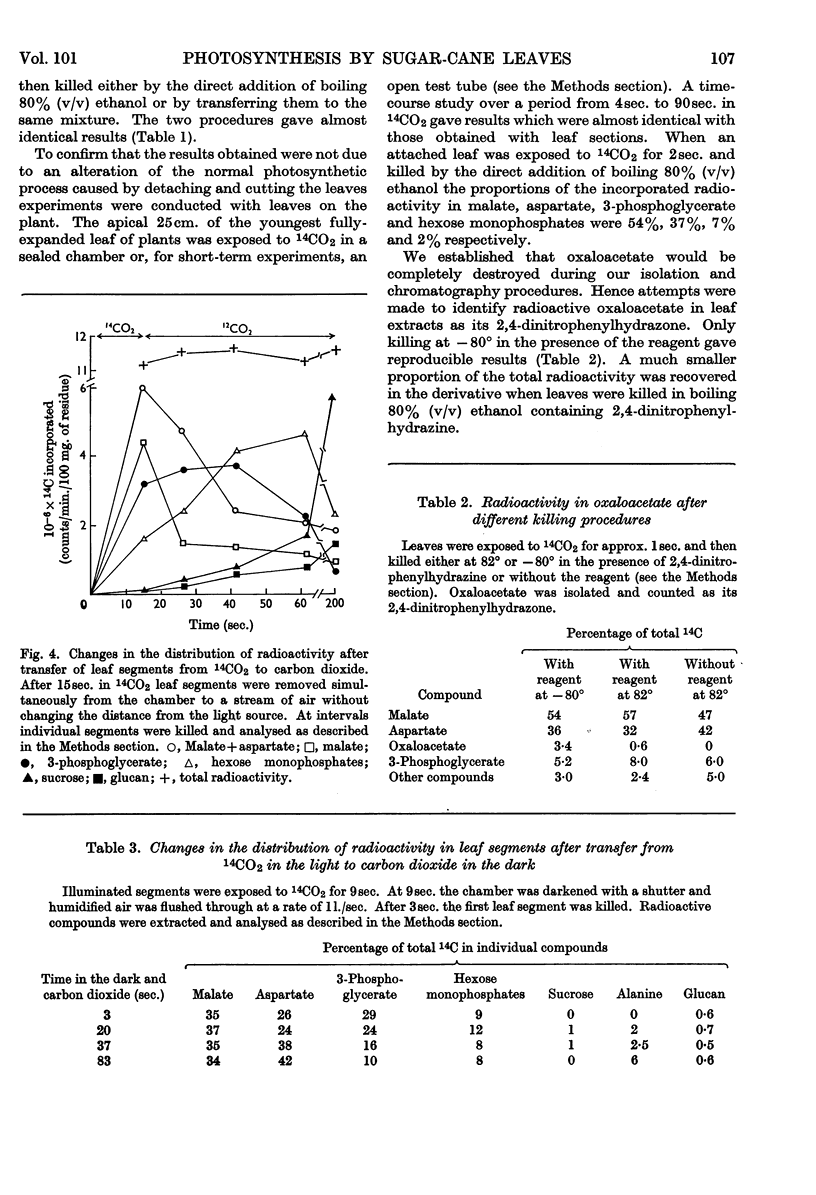
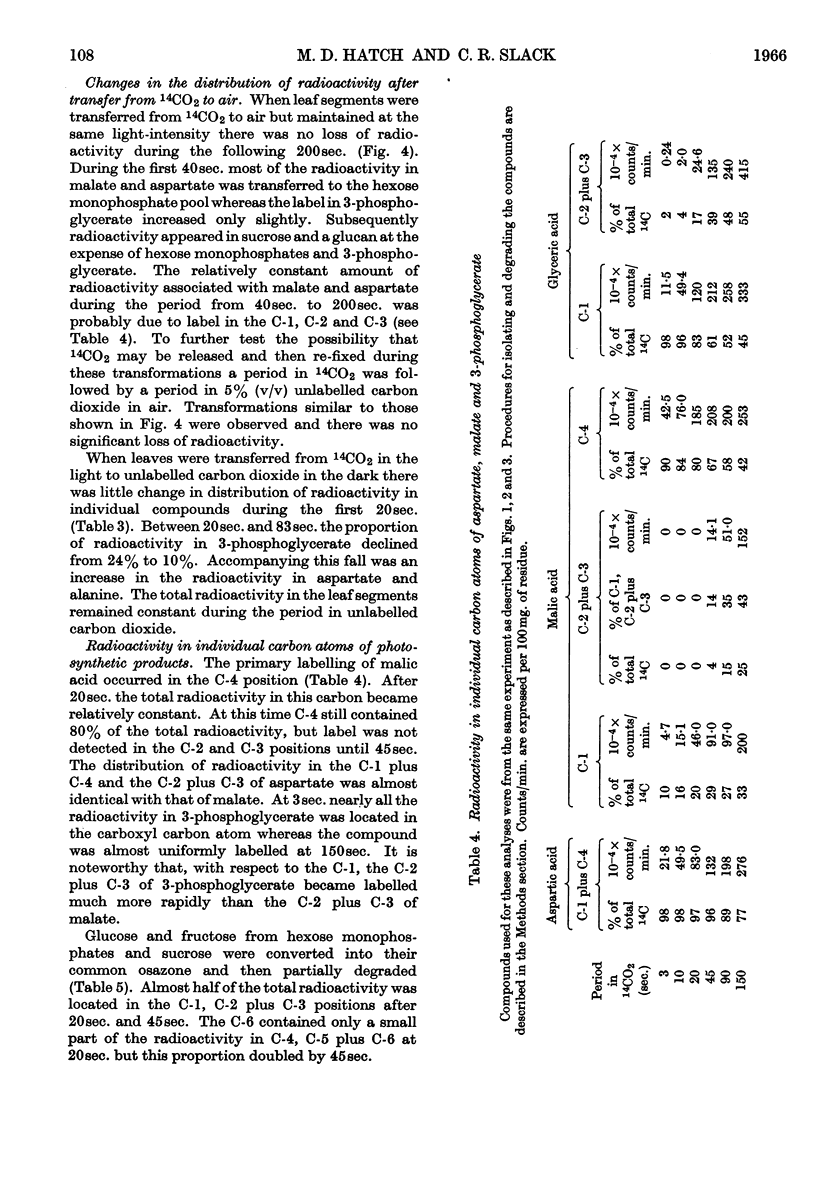
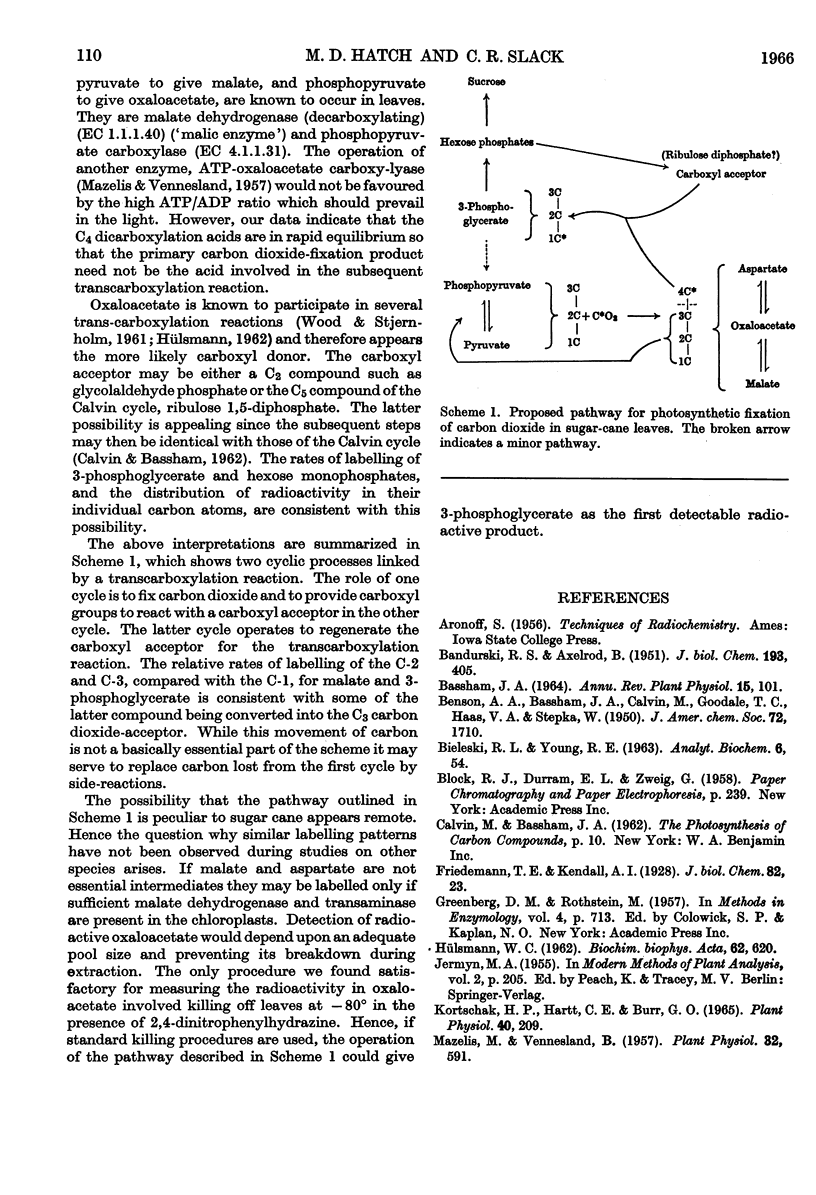
1. Radioactive products in detached leaf segments were examined after periods of steady-state photosynthesis in 14CO2. 2. After exposure to 14CO2 for approx. 1sec. more than 93% of the fixed radioactivity was located in malate, aspartate and oxaloacetate. After longer periods large proportions of the radioactivity appeared in 3-phosphoglycerate, hexose monophosphates and sucrose. Similar results were obtained with leaves still attached to the plant. 3. Radioactivity appeared first in C-4 of the dicarboxylic acids and C-1 of 3-phosphoglycerate. The labelling pattern in hexoses was consistent with their formation from 3-phosphoglycerate. 4. The reaction giving rise to C4 dicarboxylic acid appears to be the only quantitatively significant carboxylation reaction. 5. Evidence is provided that the radioactivity incorporated into the C4 dicarboxylic acid pool is transferred to sugars via 3-phosphoglycerate. A scheme is proposed to account for these observations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- Kortschak H. P., Hartt C. E., Burr G. O. Carbon Dioxide Fixation in Sugarcane Leaves. Plant Physiol. 1965 Mar;40(2):209–213. doi: 10.1104/pp.40.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelis M., Vennesland B. Carbon Dioxide Fixation into Oxalacetate in Higher Plants. Plant Physiol. 1957 Nov;32(6):591–600. doi: 10.1104/pp.32.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A., Racker E. The reductive pentose phosphate cycle. III. Enzyme activities in cell-free extracts of photosynthetic organisms. Plant Physiol. 1961 Jul;36(4):409–414. doi: 10.1104/pp.36.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. The reductive pentose phosphate cycle. I. Phosphoribulokinase and ribulose diphosphate carboxylase. Arch Biochem Biophys. 1957 Jul;69:300–310. doi: 10.1016/0003-9861(57)90496-4. [DOI] [PubMed] [Google Scholar]
- RACUSEN D. W., ARONOFF S. Metabolism of soybean leaves. V. The dark reactions following photosynthesis. Arch Biochem Biophys. 1953 Jan;42(1):25–40. doi: 10.1016/0003-9861(53)90234-3. [DOI] [PubMed] [Google Scholar]
- WOOD H. G., STJERNHOLM R. Transcarboxylase. II. Purification and properties of methylmalonyl-oxaloacetic transcarboxylase. Proc Natl Acad Sci U S A. 1961 Mar 15;47:289–303. doi: 10.1073/pnas.47.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELITCH I. THE RELATION OF GLYCOLIC ACID SYNTHESIS TO THE PRIMARY PHOTOSYNTHETIC CARBOXYLATION REACTION IN LEAVES. J Biol Chem. 1965 May;240:1869–1876. [PubMed] [Google Scholar]


