Abstract
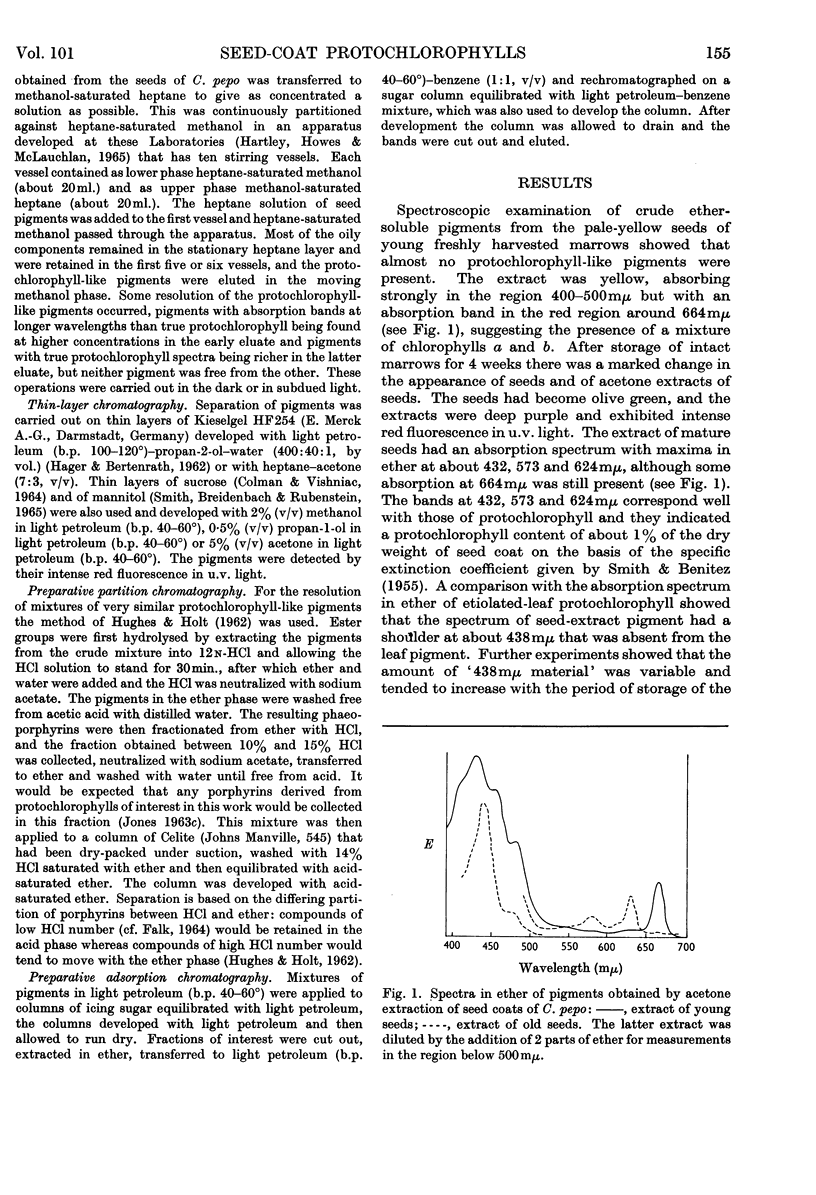
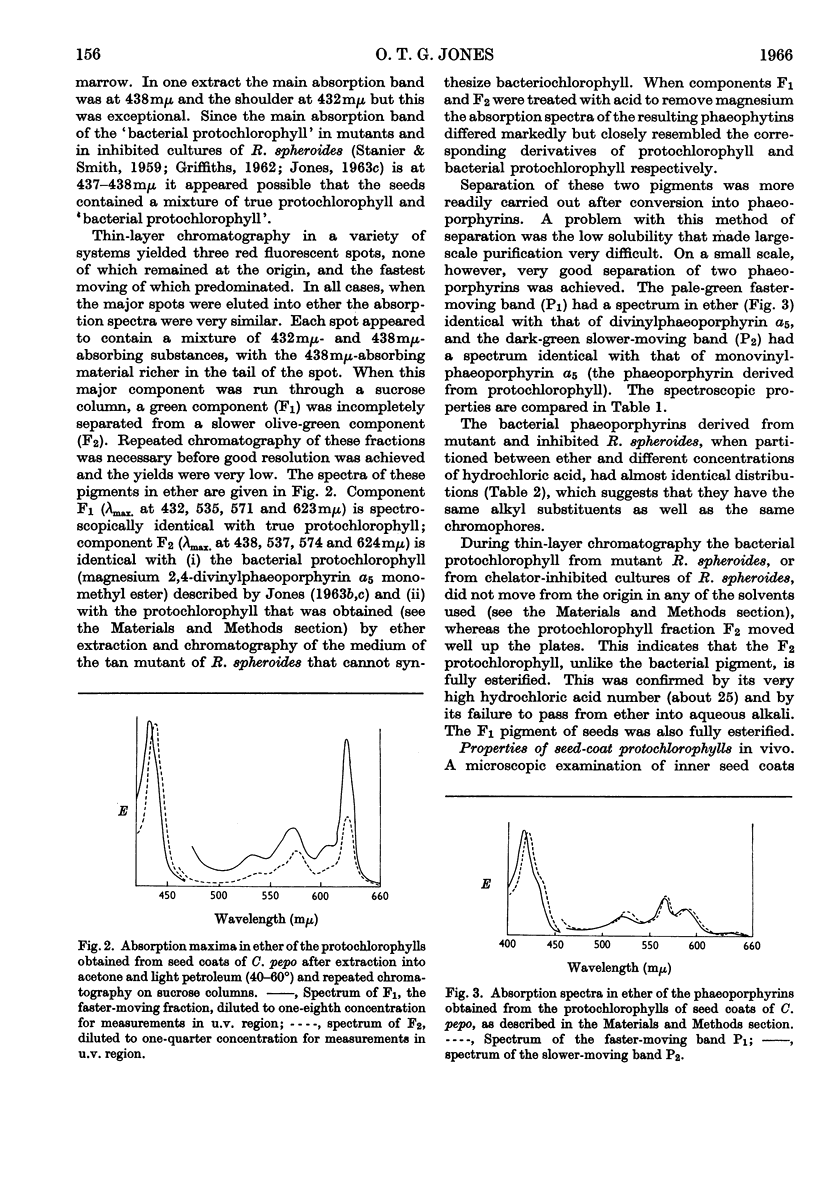
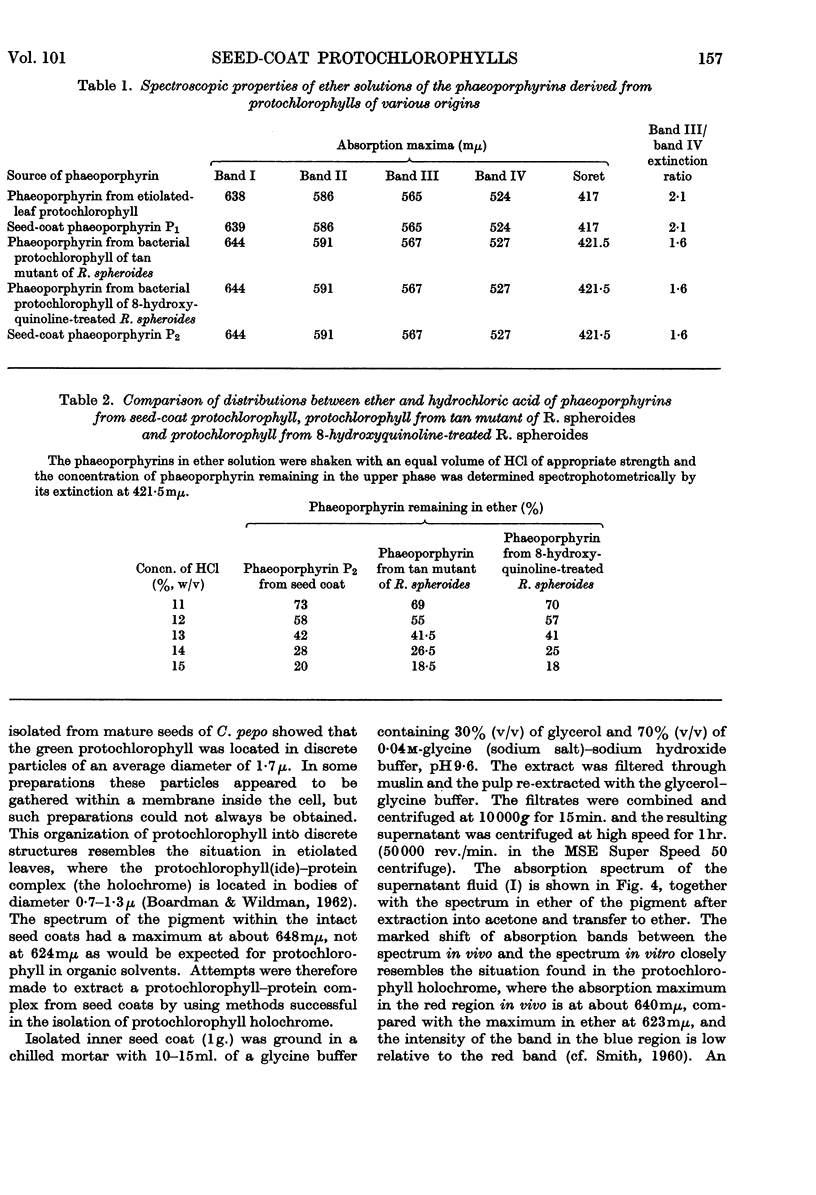
1. The inner seed coats of Cucurbita pepo were extracted with aqueous acetone and found to contain pigments with spectra similar to that of protochlorophyll. 2. When the fruits of C. pepo were stored the amount of protochlorophyll-like material in the inner seed coats increased and a form of protochlorophyll absorbing at longer wavelength was apparently formed. 3. The pigment was resolved into two forms of protochlorophyll by chromatography on sugar columns. One form with absorption maxima in ether at 432, 535, 571 and 623mμ was spectroscopically identical with plant protochlorophyll; the other, with absorption maxima at 438, 537, 574 and 624mμ, was spectroscopically identical with bacterial protochlorophyll isolated from the tan mutant of Rhodopseudomonas spheroides. The two phaeoporphyrins obtained from the seed-coat pigments closely resemble the corresponding phaeoporphyrin derivatives of plant protochlorophyll and bacterial protochlorophyll in spectroscopic and partition properties. 4. The pigment in the cells of inner seed coat of C. pepo is concentrated in discrete particles of about 1·7μ diameter. Extracts of the seed coats in a glycerol–glycine buffer were similar in spectroscopic properties to the crude protochlorophyll holochrome, but were not light-transformable. 5. After partial purification of the glycerol–glycine buffer extracts a pigment–protein complex was obtained with absorption maxima at considerably longer wavelengths than in organic solvents. 6. Preparations of the seed-coat protochlorophyll, in the presence of bovine serum albumin, adsorbed on filter paper or in colloidal solution, did not have absorption bands shifted so far to the red region as the natural protein complex isolated from the seed coat. 7. It is suggested that bacterial protochlorophyll (magnesium 2,4-divinylphaeoporphyrin a5 methyl ester) is involved in the biosynthesis of chlorophyll in both plants and photosynthetic bacteria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOARDMAN N. K. Studies on a protochlorophyll-protein complex. I. Purification and molecular-weight determination. Biochim Biophys Acta. 1962 Jul 30;62:63–79. doi: 10.1016/0006-3002(62)90492-4. [DOI] [PubMed] [Google Scholar]
- BOARDMAN N. K., WILDMAN S. G. Identification of proplastids by fluorescence microscopy and their isolation and purification. Biochim Biophys Acta. 1962 May 7;59:222–224. doi: 10.1016/0006-3002(62)90717-5. [DOI] [PubMed] [Google Scholar]
- BULL M. J., LASCELLES J. The association of protein synthesis with formation of pigments in some photosynthetic bacteria. Biochem J. 1963 Apr;87:15–28. doi: 10.1042/bj0870015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S. S., Brody M. An experiment showing that P700 can be an aggregated form of chlorophyll a. Arch Biochem Biophys. 1965 Jun;110(3):583–585. doi: 10.1016/0003-9861(65)90453-4. [DOI] [PubMed] [Google Scholar]
- COLMAN B., VISHNIAC W. SEPARATION OF CHLOROPLAST PIGMENTS ON THIN LAYERS OF SUCROSE. Biochim Biophys Acta. 1964 Mar 16;82:616–618. doi: 10.1016/0304-4165(64)90457-x. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., TAIT G. H. STUDIES ON THE BIOSYNTHESIS OF PORPHYRIN AND BACTERIOCHLOROPHYLL BY RHODOPSEUDOMONAS SPHEROIDES. 4. S-ADENOSYLMETHIONINEMAGNESIUM PROTOPORPHYRIN METHYLTRANSFERASE. Biochem J. 1963 Aug;88:325–334. doi: 10.1042/bj0880325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFITHS M. Further mutational changes in the photosynthetic pigment system of Rhodopseudomonas spheroides. J Gen Microbiol. 1962 Mar;27:427–435. doi: 10.1099/00221287-27-3-427. [DOI] [PubMed] [Google Scholar]
- JONES O. T. MAGNESIUM 2,4-DIVINYLPHAEOPORPHYRIN A5 MONOMETHYL ESTER, A PROTOCHLOROPHYLL-LIKE PIGMENT PRODUCED BY RHODOPSEUDOMONAS SPHEROIDES. Biochem J. 1963 Nov;89:182–189. doi: 10.1042/bj0890182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES O. T. THE INHIBITION OF BACTERIOCHLOROPHYLL BIOSYNTHESIS IN RHODOPSEUDOMONAS SPHEROIDES BY 8-HYDROXYQUINOLINE. Biochem J. 1963 Aug;88:335–343. doi: 10.1042/bj0880335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. T. Studies on the structure of a pigment related to chlorophyll a produced by Rhodopseudomonas spheroides. Biochem J. 1964 Jun;91(3):572–576. doi: 10.1042/bj0910572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSKI V. M., FRENCH C. S., SMITH J. H. C. The action spectrum for the transformation of protochlorophyll to chlorophyll a in normal and albino corn seedlings. Arch Biochem Biophys. 1951 Mar;31(1):1–17. doi: 10.1016/0003-9861(51)90178-6. [DOI] [PubMed] [Google Scholar]
- KRASNOVSKII A. A., KOSOBUTSKAIA L. M. Razlichnye sostoianiia khlorofilla v list'iakh rastenii. Dokl Akad Nauk SSSR. 1953 Jul 11;91(2):343–346. [PubMed] [Google Scholar]
- RYLE A. P. PEPSINOGEN B: THE ZYMOGEN OF PEPSIN B. Biochem J. 1965 Jul;96:6–16. doi: 10.1042/bj0960006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R., GRIFFITHS M., STANIER R. Y. A note on the porphyrins excreted by the blue-green mutant of Rhodopseudomonas spheroides. J Cell Physiol. 1956 Dec;48(3):459–472. doi: 10.1002/jcp.1030480308. [DOI] [PubMed] [Google Scholar]
- Smith L. W., Breidenbach R. W., Rubenstein D. Thin-Layer Chromatography of Plant Pigments on Mannitol or Sucrose. Science. 1965 Apr 23;148(3669):508–509. doi: 10.1126/science.148.3669.508. [DOI] [PubMed] [Google Scholar]


