Abstract
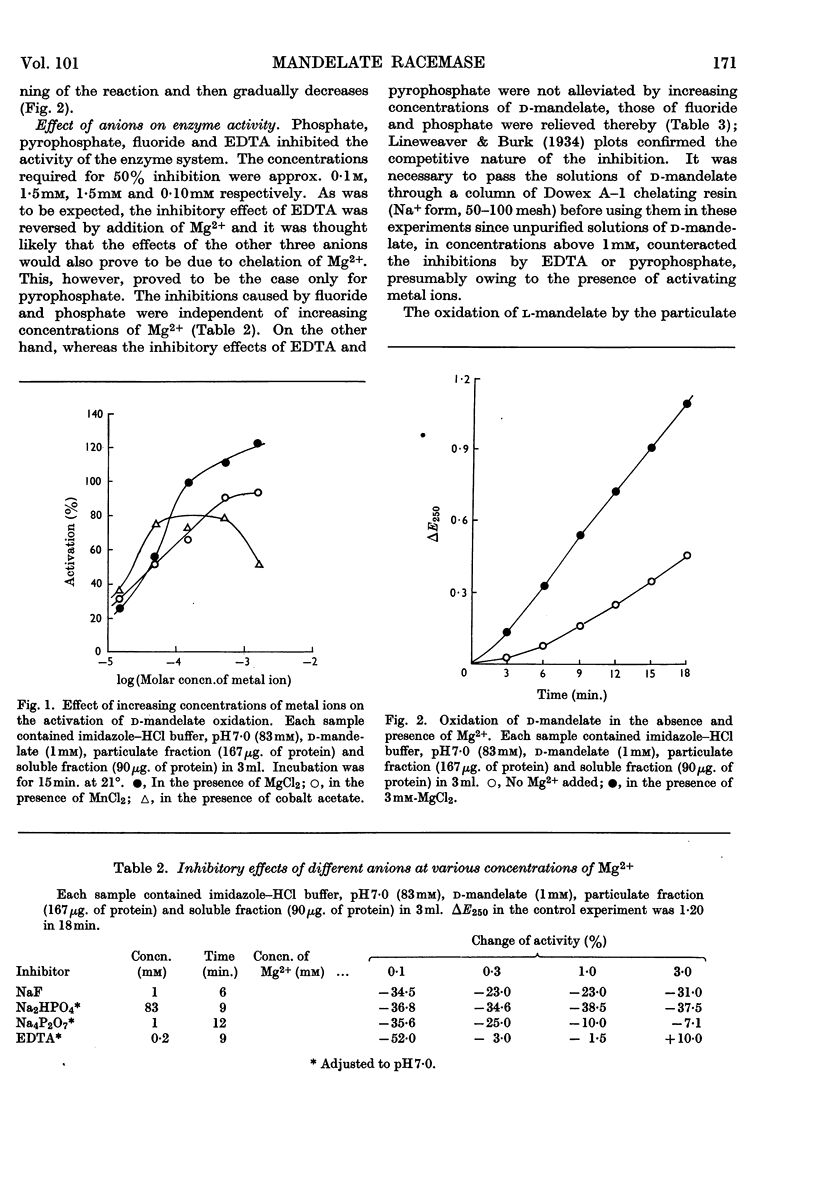
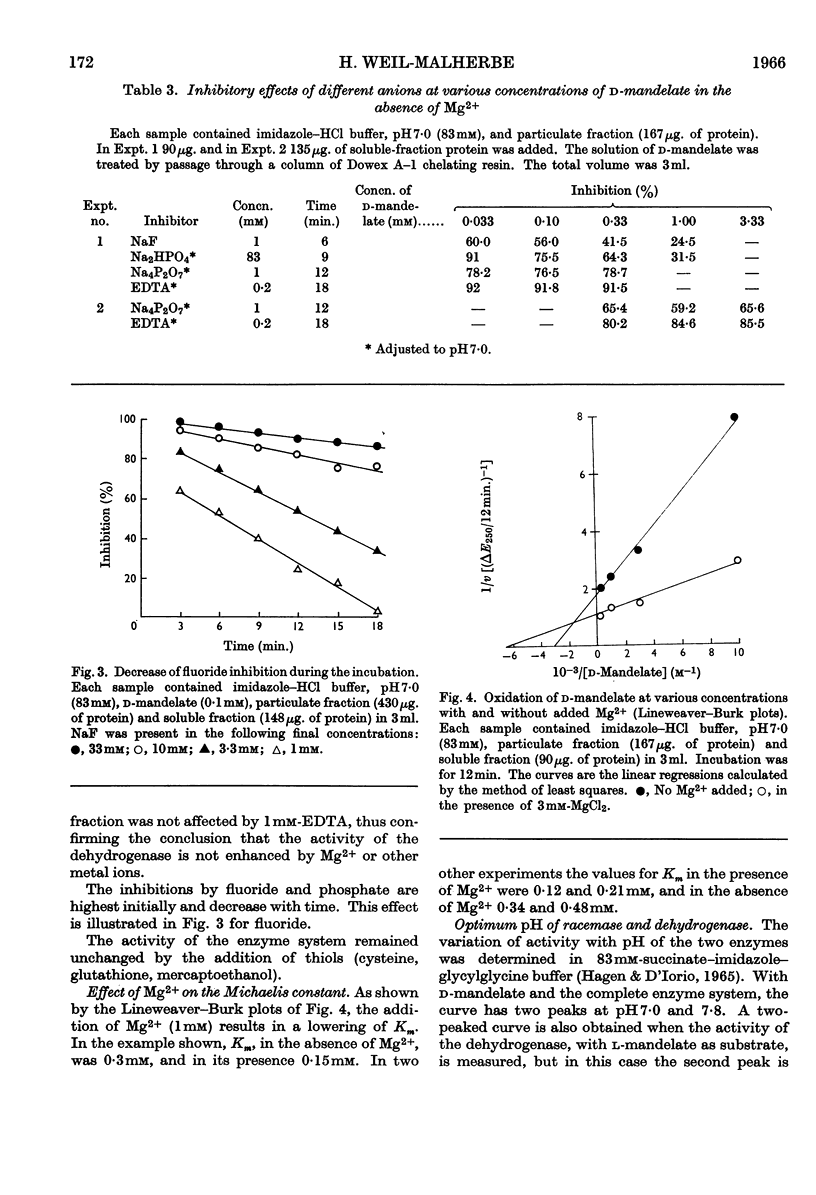
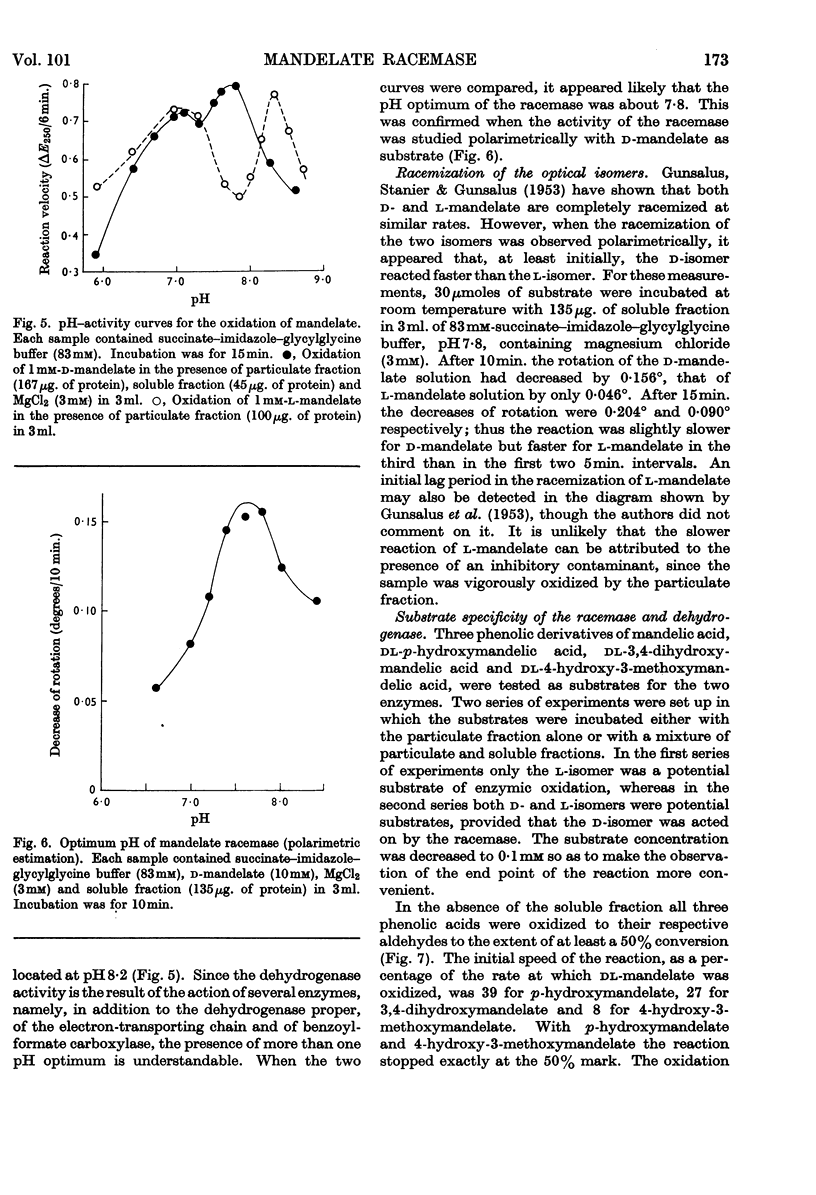
1. l-Mandelate dehydrogenase and mandelate racemase were partially purified from extracts of Pseudomonas fluorescens A–312 grown in media containing d-mandelate. 2. The activity of mandelate racemase, but not that of l-mandelate dehydrogenase, is greatly stimulated by Mg2+, Mn2+, Co2+ and, though less effectively, by Ni2+. Other metal ions are inactive or inhibitory. 3. Racemase activity is inhibited by phosphate, fluoride, pyrophosphate and EDTA. The inhibitions by pyrophosphate and EDTA are competitive with respect to the metal ion activator; those by phosphate and fluoride are competitive with respect to the substrate. 4. The addition of Mg2+ diminishes the Michaelis constant of racemase. 5. The pH optimum of the racemase is at 7·8. The pH–activity curve of the dehydrogenase complex of enzymes has two peaks, at 7·0 and 8·2. 6. The enzymic racemization of d-mandelate is initially faster than that of l-mandelate. 7. The rates of oxidation of related substrates, catalysed by l-mandelate dehydrogenase, are in the decreasing order: l-p-hydroxymandelate; l-3,4-dihydroxymandelate; l-4-hydroxy-3-methoxymandelate. The racemase is active towards d-p-hydroxymandelate but inactive towards d-3,4-dihydroxymandelate and d-4-hydroxy-3-methoxymandelate. Since 4-hydroxy-3-methoxymandelate, and presumably also 3,4-dihydroxymandelate, arising from the metabolism of catechol-amines, have the d-configuration, the enzymes studied cannot be utilized for estimation of the last two acids in urine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG M. D., McMILLAN A., SHAW K. N. 3-Methoxy-4-hydroxy-D-mandelic acid, a urinary metabolite of norepinephrine. Biochim Biophys Acta. 1957 Aug;25(2):422–423. doi: 10.1016/0006-3002(57)90491-2. [DOI] [PubMed] [Google Scholar]
- GUNSALUS C. F., STANIER R. Y., GUNSALUS I. C. The enzymatic conversion of mandelic acid to benzoic acid. III. Fractionation and properties of the soluble enzymes. J Bacteriol. 1953 Nov;66(5):548–553. doi: 10.1128/jb.66.5.548-553.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen P., D'Iorio A. Studies with the ATPase of adrenal medulla. Can J Biochem. 1965 Oct;43(10):1633–1642. doi: 10.1139/o65-180. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORRISON J. F., GRIFFITHS D. E., ENNOR A. H. The purification and properties of arginine phosphokinase. Biochem J. 1957 Jan;65(1):143–153. doi: 10.1042/bj0650143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenburgh M. S., Snoswell A. M. Use of streptomycin in the separation of nucleic acids from protein in a bacterial extract. Nature. 1965 Sep 25;207(5004):1416–1417. doi: 10.1038/2071416a0. [DOI] [PubMed] [Google Scholar]
- ROSANO C. L. ENZYMATIC METHOD FOR DETERMINATION OF VANILLYL MANDELIC ACID. Clin Chem. 1964 Aug;10:673–677. [PubMed] [Google Scholar]
- STANIER R. Y. Problems of bacterial oxidative metabolism. Bacteriol Rev. 1950 Sep;14(3):179–191. doi: 10.1128/br.14.3.179-191.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil-Malherbe H., Smith E. R. The estimation of metanephrine, normetanephrine, and 3,4-dihydroxymandelic acid in urine. Pharmacol Rev. 1966 Mar;18(1):331–341. [PubMed] [Google Scholar]


