Abstract
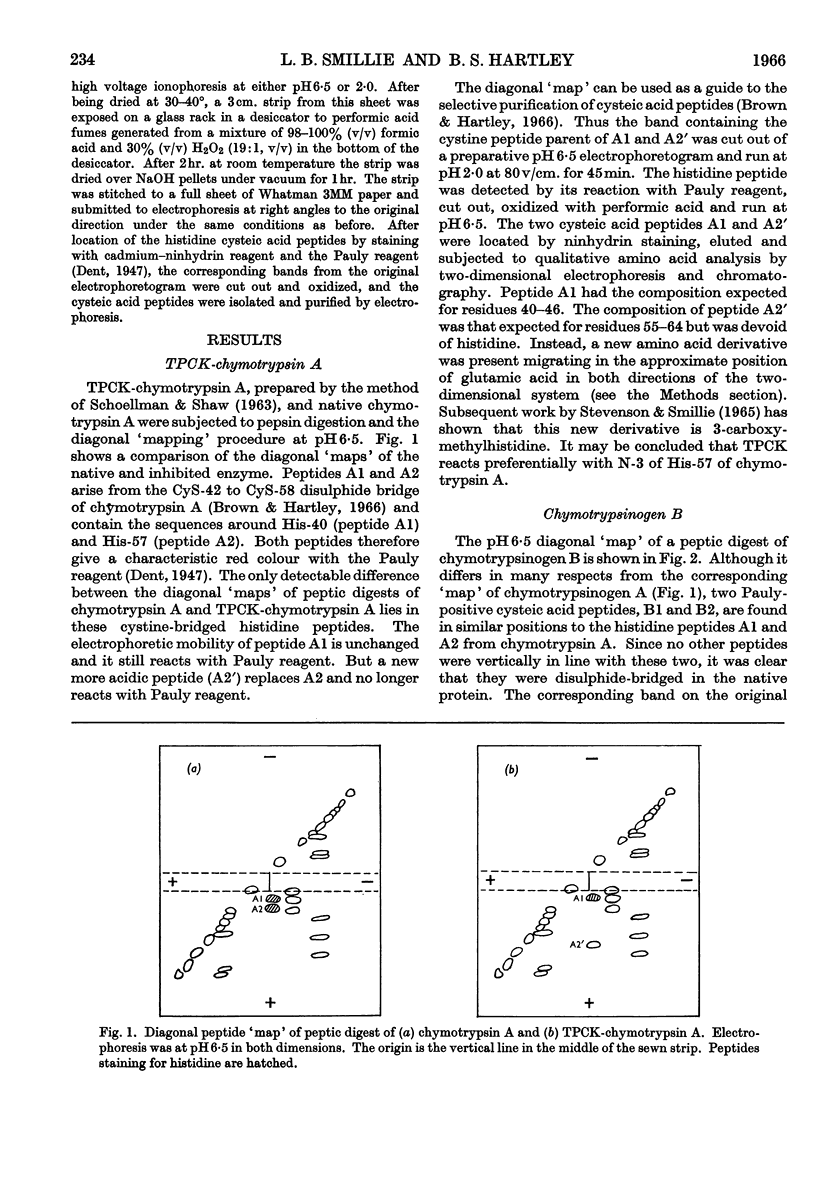
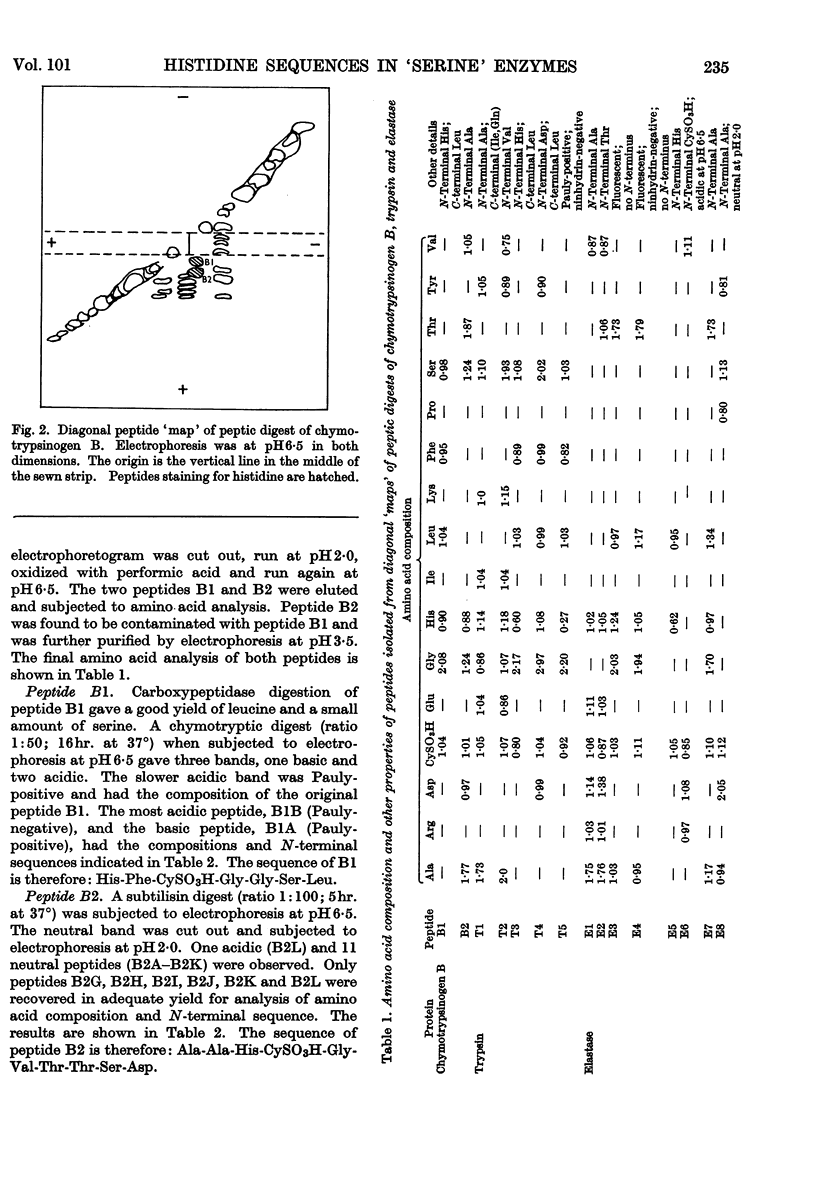
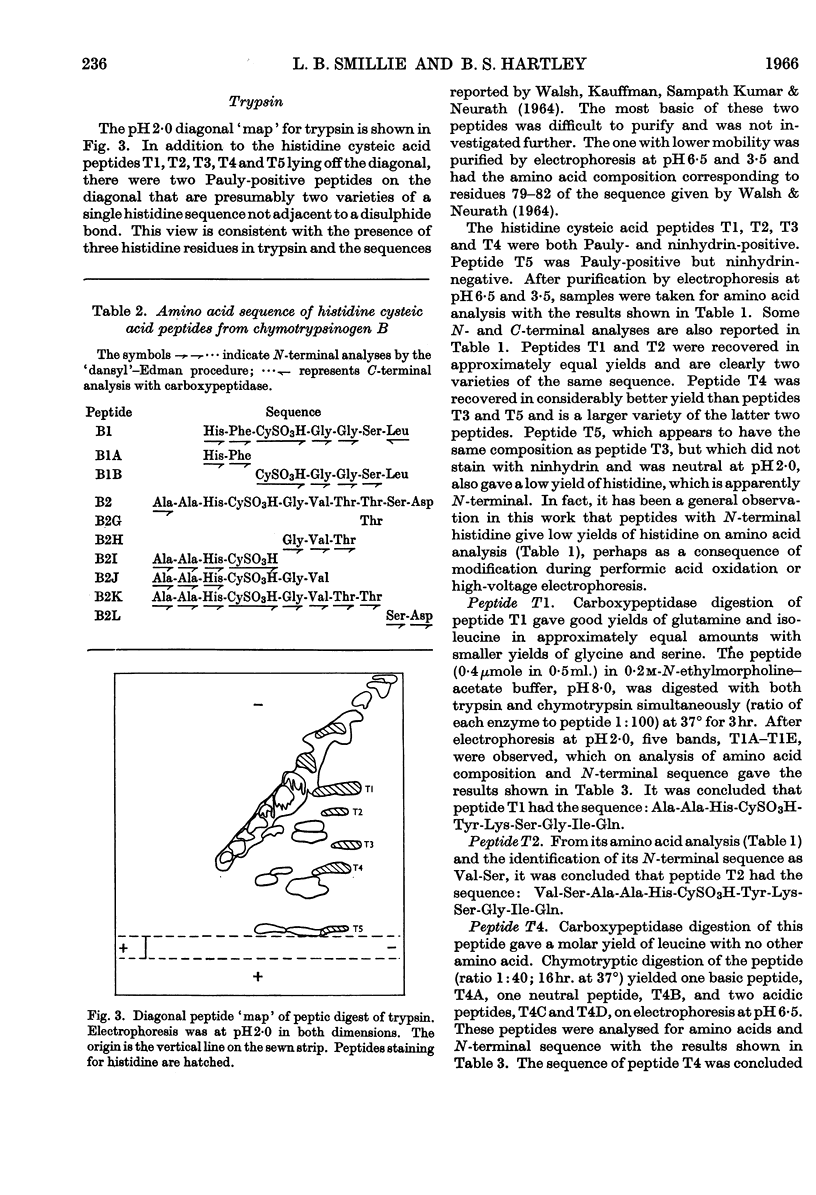
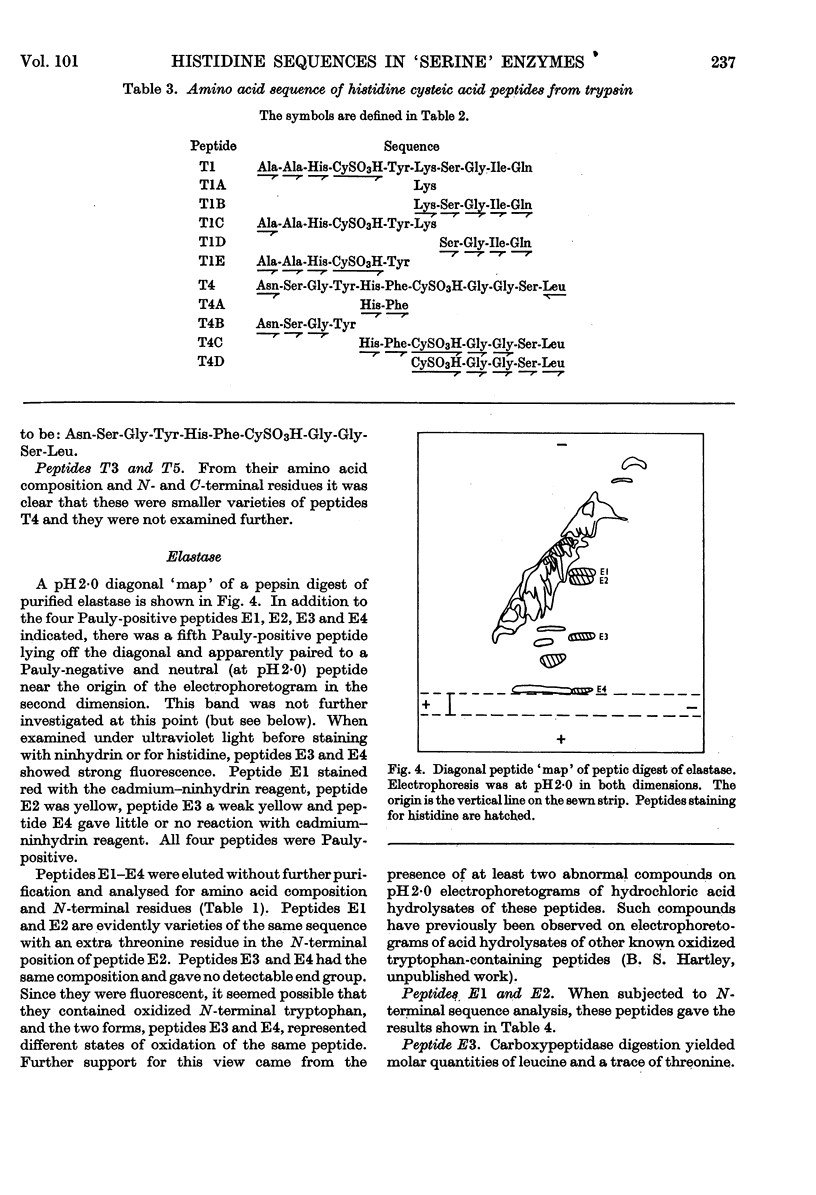
1. A comparison of the diagonal `maps' of chymotrypsin A and `tosylphenylalanyl chloromethyl ketone'-inhibited chymotrypsin A showed that His-57 is alkylated specifically by this substrate analogue. 2. From peptic digests of chymotrypsinogen A and B, trypsin and elastase it was demonstrated by the diagonal electrophoretic technique that a common di-histidine cystine-bridged structure is present in all four enzymes. 3. The sequences of these peptides were determined and show that the positions of the two histidine residues relative to the disulphide bond are a common feature. Thus His-40 of chymotrypsin A is only two residues removed from CyS-42, and His-57 is adjacent to the other half of this bridge, CyS-58. 4. Considerable variation in sequence occurs about His-40, where the aromatic residues 39 and 41 of the chymotrypsins and trypsin are replaced by alanine and threonine in elastase. There is a remarkable similarity in sequence following CyS-42 and preceding CyS-58 in all four enzymes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINK N. G., LEWIS U. J., WILLIAMS D. E. Pancreatic elastase: purification, properties, and function. J Biol Chem. 1956 Oct;222(2):705–720. [PubMed] [Google Scholar]
- Brown J. R., Hartley B. S. Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J. 1966 Oct;101(1):214–228. doi: 10.1042/bj1010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON G. H., KAUFFMAN D. L., NEURATH H. Amino acid sequence in the region of diisopropylphosphoryl binding in diisopropylphosphoryl-trypsin. J Biol Chem. 1958 Dec;233(6):1373–1381. [PubMed] [Google Scholar]
- Dent C. E. The amino-aciduria in Fanconi syndrome. A study making extensive use of techniques based on paper partition chromatography. Biochem J. 1947;41(2):240–253. doi: 10.1042/bj0410240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENENKEL A. G., SMILLIE L. B. PREPARATION AND PURITY OF CHYMOTRYPSINOGEN B. Biochemistry. 1963 Nov-Dec;2:1445–1448. doi: 10.1021/bi00906a042. [DOI] [PubMed] [Google Scholar]
- ENENKEL A. G., SMILLIE L. B. SPECIFICITY OF CHYMOTRYPSIN B TOWARD GLUCAGON. Biochemistry. 1963 Nov-Dec;2:1449–1454. doi: 10.1021/bi00906a043. [DOI] [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S. AMINO-ACID SEQUENCE OF BOVINE CHYMOTRYPSINOGEN-A. Nature. 1964 Mar 28;201:1284–1287. doi: 10.1038/2011284a0. [DOI] [PubMed] [Google Scholar]
- HARTLEY B. S. Proteolytic enzymes. Annu Rev Biochem. 1960;29:45–72. doi: 10.1146/annurev.bi.29.070160.000401. [DOI] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- HUNT J. A., OTTENSEN M. A comparison of three proteinases from various strains of Bacillus subtilis. Biochim Biophys Acta. 1961 Apr 1;48:411–412. doi: 10.1016/0006-3002(61)90498-x. [DOI] [PubMed] [Google Scholar]
- Hartley B. S., Brown J. R., Kauffman D. L., Smillie L. B. Evolutionary similarities between pancreatic proteolytic enzymes. Nature. 1965 Sep 11;207(5002):1157–1159. doi: 10.1038/2071157a0. [DOI] [PubMed] [Google Scholar]
- INAGAMI T., STURTEVANT J. M. Nonspecific catalyses by alpha-chymotrypsin and trypsin. J Biol Chem. 1960 Apr;235:1019–1023. [PubMed] [Google Scholar]
- INAGAMI T. THE MECHANISM OF THE SPECIFICITY OF TRYPSIN CATALYSIS. I. INHIBITION BY ALKYL AMMONIUM IONS. J Biol Chem. 1964 Mar;239:787–791. [PubMed] [Google Scholar]
- KEIL B., PRUSIK, SORM F. DISULPHIDE BRIDGES AND A SUGGESTED STRUCTURE OF CHYMOTRYPSINOGEN. Biochim Biophys Acta. 1963 Nov 15;78:559–561. doi: 10.1016/0006-3002(63)90927-2. [DOI] [PubMed] [Google Scholar]
- KOSHLAND D. E., Jr, STRUMEYER D. H., RAY W. J., Jr Amino acids involved in the action of chymotrypsin. Brookhaven Symp Biol. 1962 Dec;15:101–133. [PubMed] [Google Scholar]
- MARES-GUIA M., SHAW E. STUDIES ON THE ACTIVE CENTER OF TRYPSIN. THE BINDING OF AMIDINES AND GUANIDINES AS MODELS OF THE SUBSTRATE SIDE CHAIN. J Biol Chem. 1965 Apr;240:1579–1585. [PubMed] [Google Scholar]
- NAUGHTON M. A., SANGER F., HARTLEY B. S., SHAW D. C. The amino acid sequence around the reactive serine residue of some proteolytic enzymes. Biochem J. 1960 Oct;77:149–163. doi: 10.1042/bj0770149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAUGHTON M. A., SANGER F. Purification and specificity of pancreatic elastase. Biochem J. 1961 Jan;78:156–163. doi: 10.1042/bj0780156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONG E. B., SHAW E., SCHOELLMANN G. THE IDENTIFICATION OF THE HISTIDINE RESIDUE AT THE ACTIVE CENTER OF CHYMOTRYPSIN. J Biol Chem. 1965 Feb;240:694–698. [PubMed] [Google Scholar]
- RYLE A. P., SANGER F., SMITH L. F., KITAI R. The disulphide bonds of insulin. Biochem J. 1955 Aug;60(4):541–556. doi: 10.1042/bj0600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- WALSH K. A., KAUFFMAN D. L., KUMAR K. S., NEURATH H. ON THE STRUCTURE AND FUNCTION OF BOVINE TRYPSINOGEN AND TRYPSIN. Proc Natl Acad Sci U S A. 1964 Feb;51:301–308. doi: 10.1073/pnas.51.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSH K. A., NEURATH H. TRYPSINOGEN AND CHYMOTRYPSINOGEN AS HOMOLOGOUS PROTEINS. Proc Natl Acad Sci U S A. 1964 Oct;52:884–889. doi: 10.1073/pnas.52.4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMASAKI M., BROWN J. R., COX D. J., GREENSHIELDS R. N., WADE R. D., NEURATH H. PROCARBOXYPEPTIDASE A-S6. FURTHER STUDIES OF ITS ISOLATION AND PROPERTIES. Biochemistry. 1963 Jul-Aug;2:859–866. doi: 10.1021/bi00904a039. [DOI] [PubMed] [Google Scholar]


