Abstract
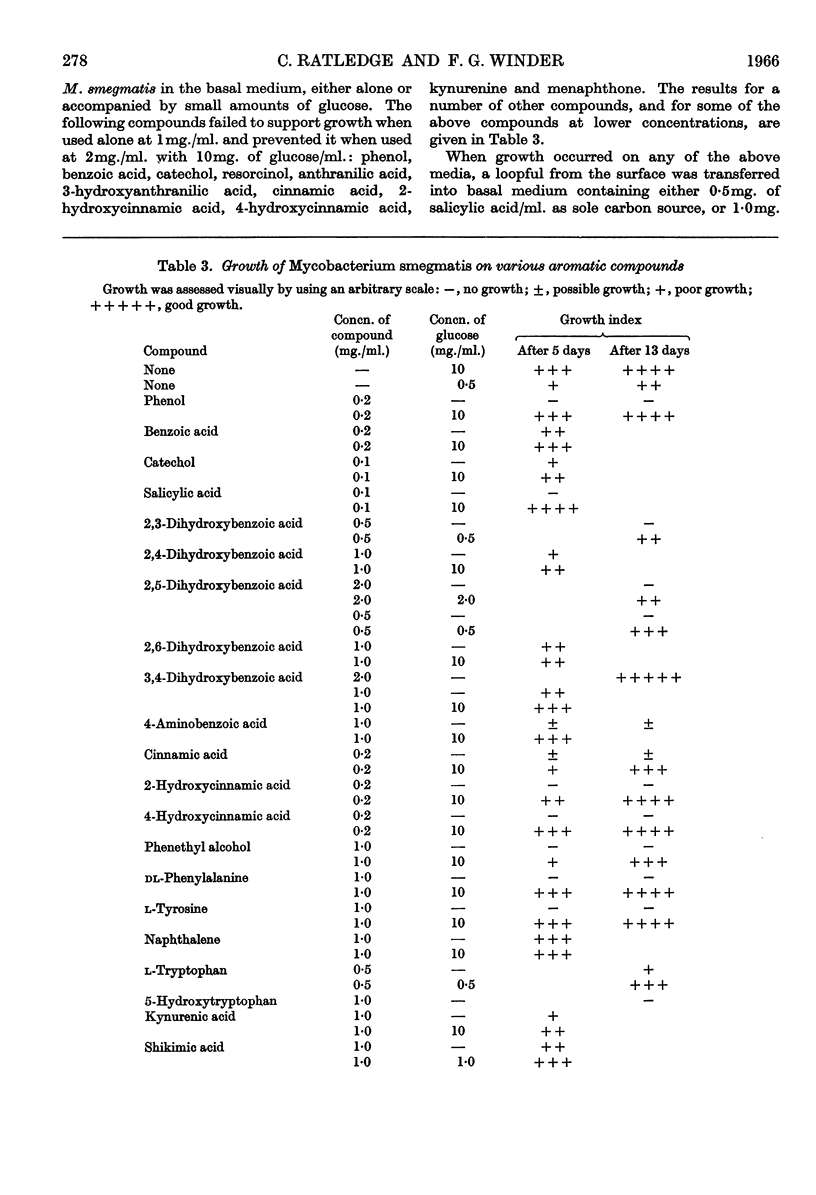
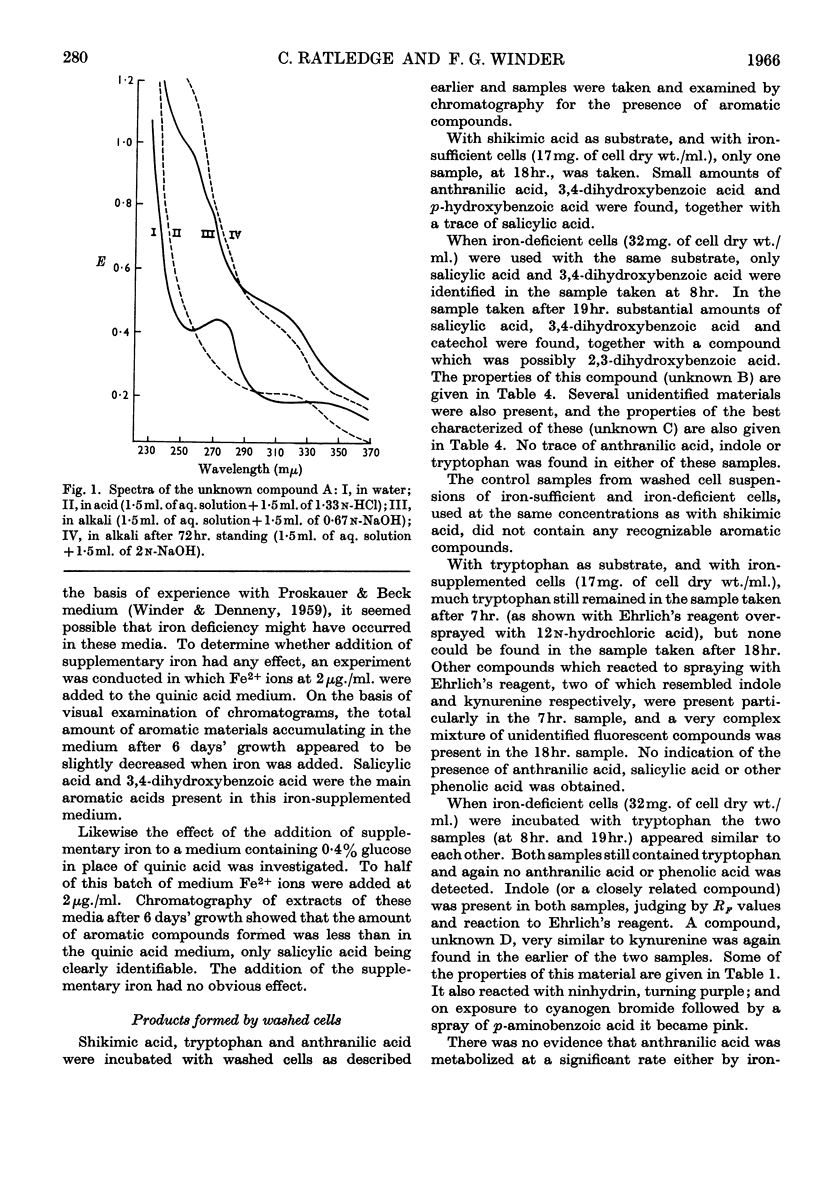
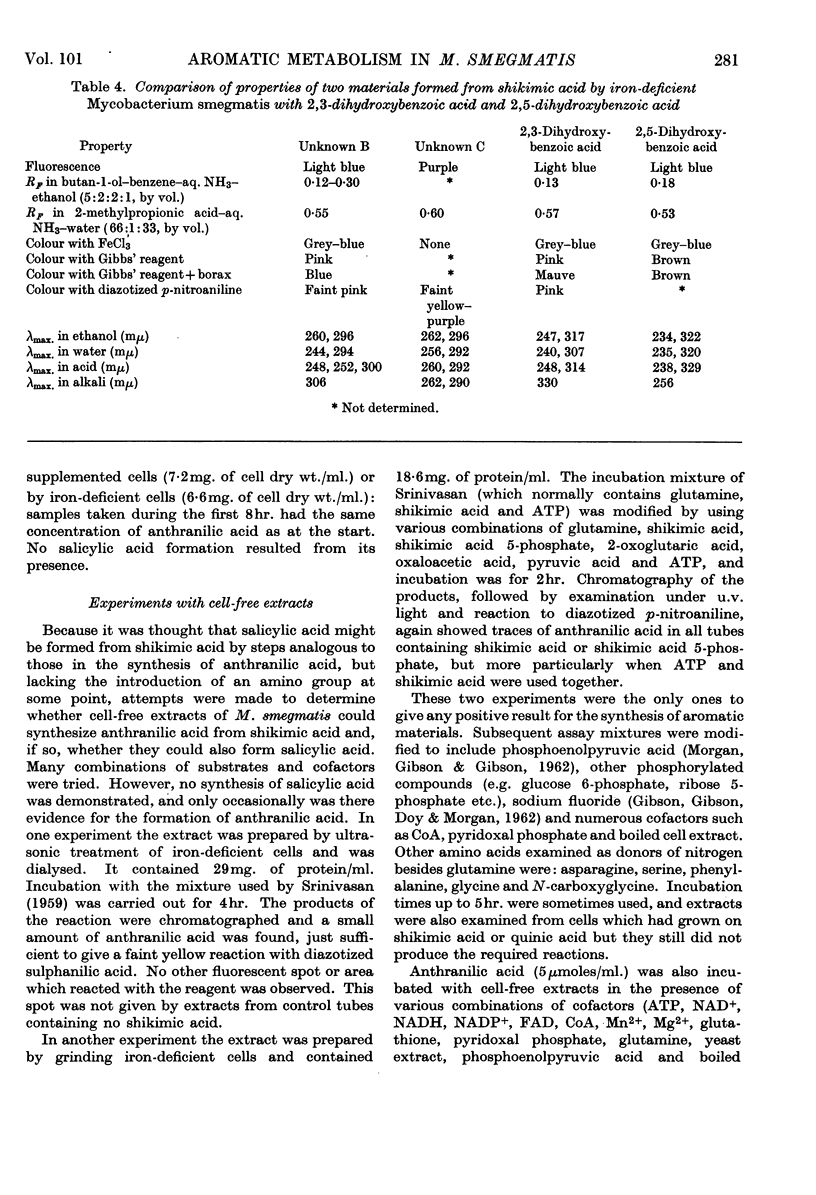
1. Although Mycobacterium smegmatis could utilize a number of aromatic compounds as sole sources of carbon for growth, it did not appear to be able to use salicylic acid for growth or to metabolize it to any great extent. 2. When M. smegmatis was grown on shikimic acid as sole source of carbon, salicylic acid, anthranilic acid and 3,4-dihydroxybenzoic acid were released into the medium. When it was grown on quinic acid these compounds, together with p-hydroxybenzoic acid, p-hydroxyphenylacetic acid and a number of unidentified compounds, were formed. When it was grown on glucose only small amounts of salicylic acid could be detected. 3. When a washed suspension of cells with a normal iron content was incubated with shikimic acid, only small amounts of aromatic compounds were formed in the medium. When the cells were iron-deficient, substantial amounts of salicylic acid, 3,4-dihydroxybenzoic acid and catechol were formed, together with several other compounds not definitely identified. 4. When washed suspensions of cells, whether iron-sufficient or iron-deficient, were incubated with tryptophan no evidence of formation of salicylic acid, anthranilic acid or phenolic compounds was obtained. Washed suspensions did not convert anthranilic acid into salicylic acid. 5. When cell-free extracts of M. smegmatis were incubated with shikimic acid, or shikimic acid 5-phosphate, traces of anthranilic acid were formed under certain conditions. No formation of salicylic acid or other phenolic compound was observed even when a number of combinations of cofactors and coenzymes were tried.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BHAT M. G., RAMAKRISHNAN T., BHAT J. V. Salicylate as intermediate in the breakdown of aromatic ring by Pseudomonas convexa var. hippuricum. Can J Microbiol. 1959 Feb;5(1):109–118. doi: 10.1139/m59-013. [DOI] [PubMed] [Google Scholar]
- Bernheim F. The Effect of Various Substances on the Oxygen Uptake of the Tubercle Bacillus. J Bacteriol. 1941 Mar;41(3):387–395. doi: 10.1128/jb.41.3.387-395.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNLEY H. N., EVANS W. C. Oxidative metabolism of polycyclic hydrocarbons by soil Pseudomonads. Nature. 1958 Aug 9;182(4632):373–375. doi: 10.1038/182373a0. [DOI] [PubMed] [Google Scholar]
- Gale G. R. THE OXIDATION OF BENZOIC ACID BY MYCOBACTERIA I. : Metabolic Pathways in Mycobacterium tuberculosis, Mycobacterium butyricum, and Mycobacterium phlei. J Bacteriol. 1952 Feb;63(2):273–278. doi: 10.1128/jb.63.2.273-278.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. I., Gibson F. Preliminary studies on the isolation and metabolism of an intermediate in aromatic biosynthesis: chorismic acid. Biochem J. 1964 Feb;90(2):248–256. doi: 10.1042/bj0900248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. I., Gibson F. Preliminary studies on the isolation and metabolism of an intermediate in aromatic biosynthesis: chorismic acid. Biochem J. 1964 Feb;90(2):248–256. doi: 10.1042/bj0900248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Kuno S., Itada N., Hayaishi O., Kozuka S., Oae S. O-18 studies on anthranilate hydroxylase--a novel mechanism of double hydroxylation. Biochem Biophys Res Commun. 1964 Aug 11;16(6):556–561. doi: 10.1016/0006-291x(64)90192-5. [DOI] [PubMed] [Google Scholar]
- MASON H. S. OXIDASES. Annu Rev Biochem. 1965;34:595–634. doi: 10.1146/annurev.bi.34.070165.003115. [DOI] [PubMed] [Google Scholar]
- MCGEER E. G., ROBERTSON M. C., MCGEER P. L. Chromatographic characteristics of some aromatic compounds of biological interest. Can J Biochem Physiol. 1961 Mar;39:605–627. doi: 10.1139/o61-060. [DOI] [PubMed] [Google Scholar]
- PITTARD A. J., GIBSON F., DOY C. H. A possible relationship between the formation of o-dihydric phenols and tryptophan biosynthesis by Aerobacter aerogens. Biochim Biophys Acta. 1962 Feb 26;57:290–298. doi: 10.1016/0006-3002(62)91122-8. [DOI] [PubMed] [Google Scholar]
- RATLEDGE C. RELATIONSHIP BETWEEN THE PRODUCTS OF AROMATIC BIOSYNTHESIS IN MYCOBACTERIUM SMEGMATIS AND AEROBACTER AEROGENES. Nature. 1964 Jul 25;203:428–429. doi: 10.1038/203428a0. [DOI] [PubMed] [Google Scholar]
- RATLEDGE C., WINDER F. G. The accumulation of salicylic acid by mycobacteria during growth on an iron-deficient medium. Biochem J. 1962 Sep;84:501–506. doi: 10.1042/bj0840501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWINCK I., ADAMS E. Aromatic biosynthesis. XVI. Aromatization of prephenic acid to p-hydroxyphenylpyruvic acid, a step in tyrosine biosynthesis in Escherichia coli. Biochim Biophys Acta. 1959 Nov;36:102–117. doi: 10.1016/0006-3002(59)90074-5. [DOI] [PubMed] [Google Scholar]
- SNOW G. A. THE STRUCTURE OF MYCOBACTIN P, A GROWTH FACTOR FOR MYCOBACTERIUM JOHNEI, AND THE SIGNIFICANCE OF ITS IRON COMPLEX. Biochem J. 1965 Jan;94:160–165. doi: 10.1042/bj0940160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRINIVASAN P. R., RIVERA A., Jr THE ENZYMATIC SYNTHESIS OF ANTHRANILATE FROM SHIKIMATE 5-PHOSPHATE AND L-GLUTAMINE. Biochemistry. 1963 Sep-Oct;2:1059–1062. doi: 10.1021/bi00905a025. [DOI] [PubMed] [Google Scholar]
- Snow G. A. Isolation and structure of mycobactin T, a growth factor from Mycobacterium tuberculosis. Biochem J. 1965 Oct;97(1):166–175. doi: 10.1042/bj0970166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANIUCHI H., HATANAKA M., KUNO S., HAYAISHI O., NAKAJIMA M., KURIHARA N. ENZYMATIC FORMATION OF CATECHOL FROM ANTHRANILIC ACID. J Biol Chem. 1964 Jul;239:2204–2211. [PubMed] [Google Scholar]
- VOETS J. P. LE M'ETABOLISME DU BENZOATE PAR AZOTOBACTER VINELANDII. Ann Inst Pasteur (Paris) 1963 Aug;105:383–391. [PubMed] [Google Scholar]
- WILKINS J. H., BARNES J. H. Effects of phencyclidine on the radiosensitivity of mice. Nature. 1962 Sep 22;195:1173–1175. [PubMed] [Google Scholar]
- WINDER F., DENNENY J. M. Effect of iron and zinc on nucleic acid and protein synthesis in Mycobacterium smegmatis. Nature. 1959 Aug 29;184(Suppl 10):742–743. doi: 10.1038/184742a0. [DOI] [PubMed] [Google Scholar]
- Winder F. G., Brennan P., Ratledge C. Synthesis of fatty acids by extracts of mycobacteria and the absence of inhibition by isoniazid. Biochem J. 1964 Dec;93(3):635–640. doi: 10.1042/bj0930635. [DOI] [PMC free article] [PubMed] [Google Scholar]


