Abstract
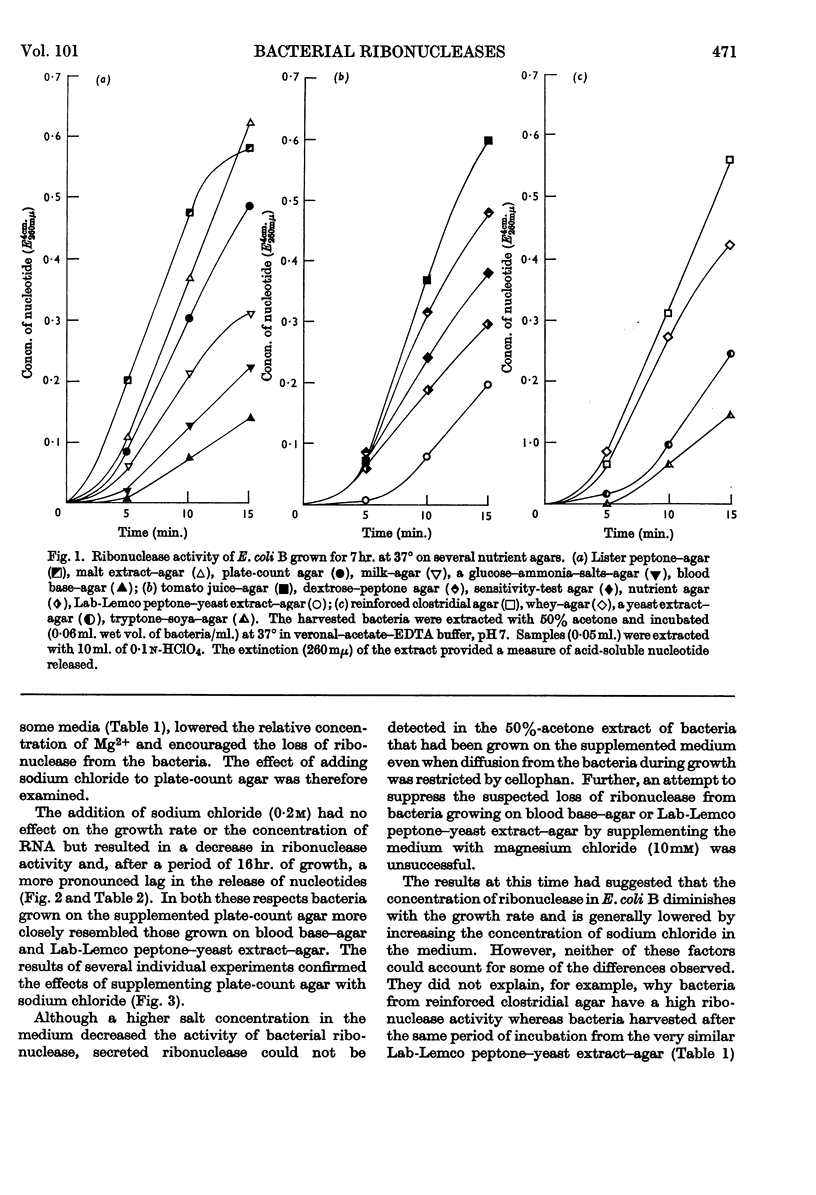
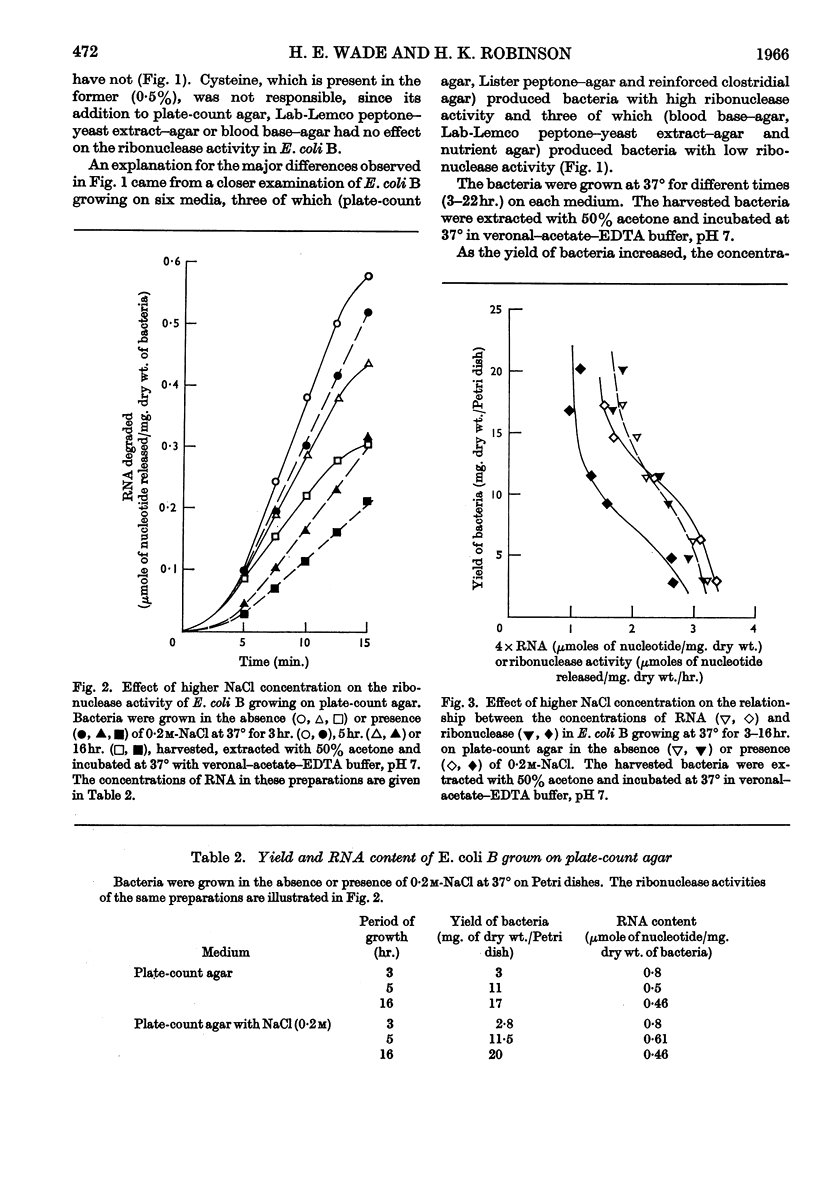
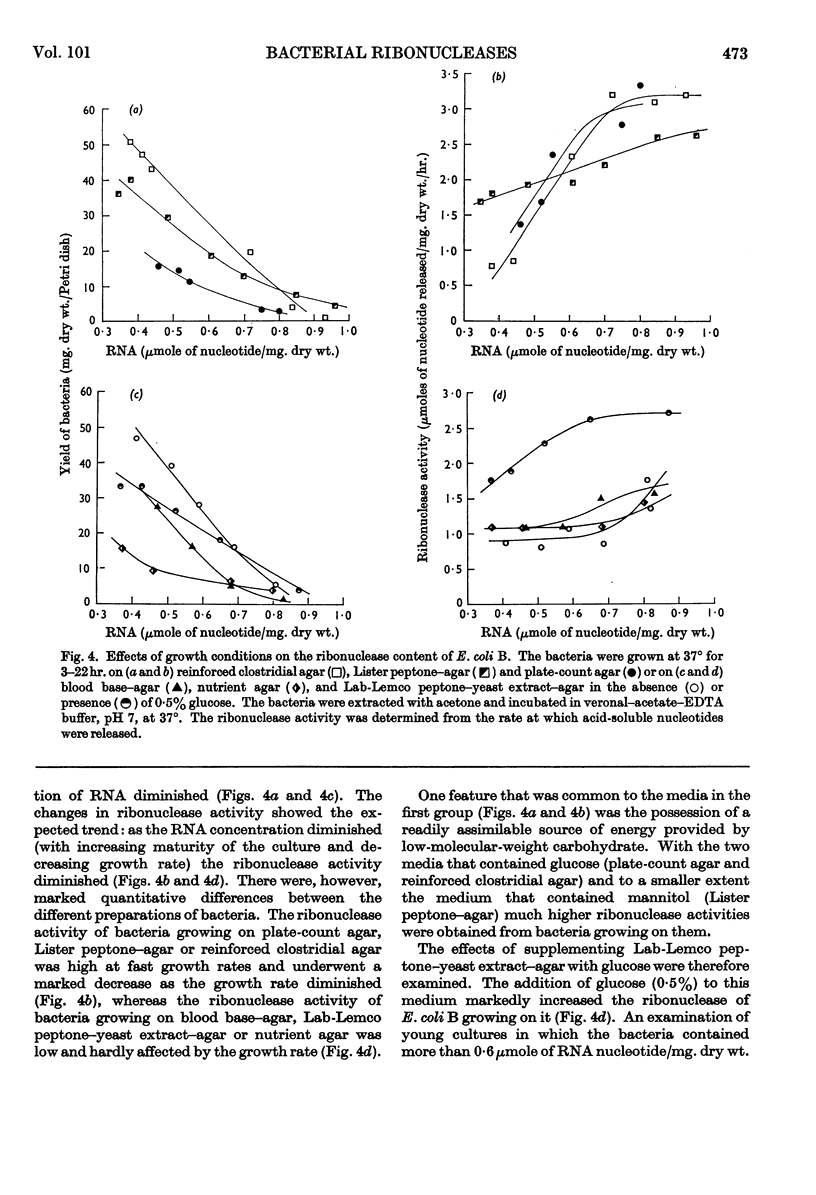
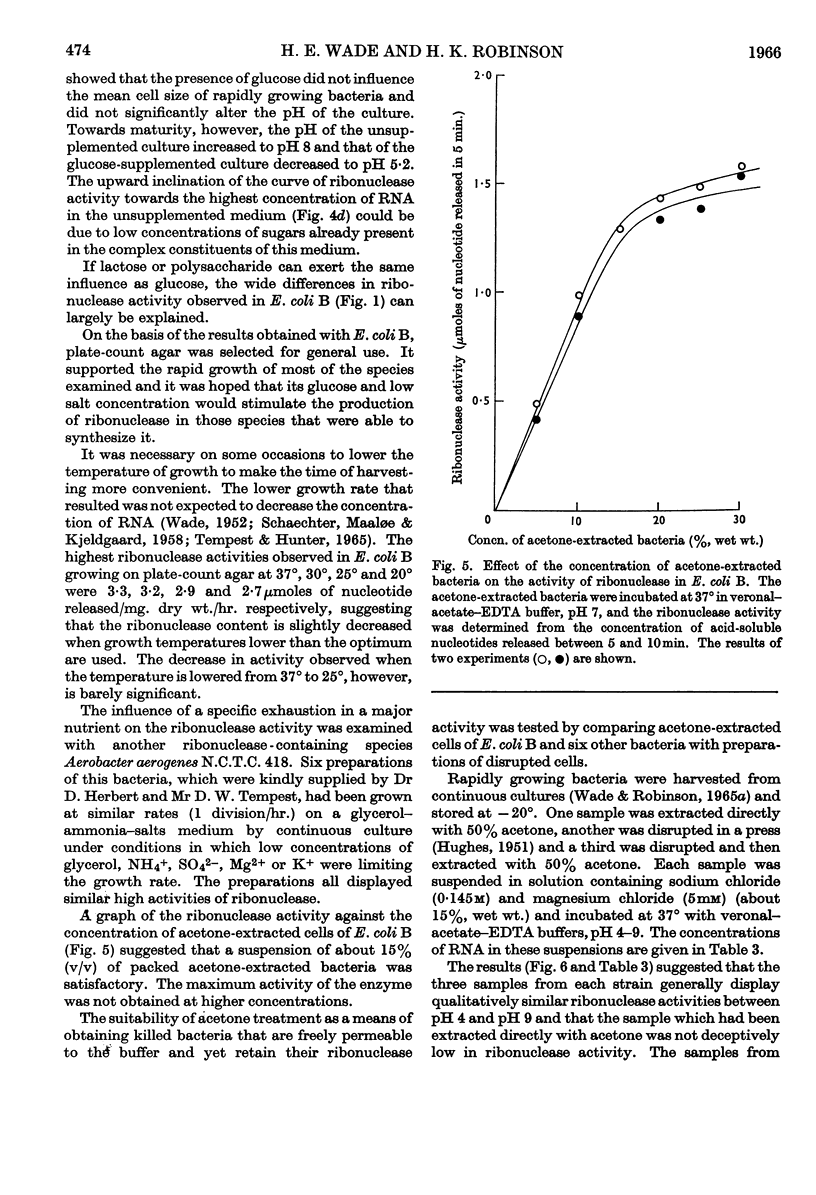
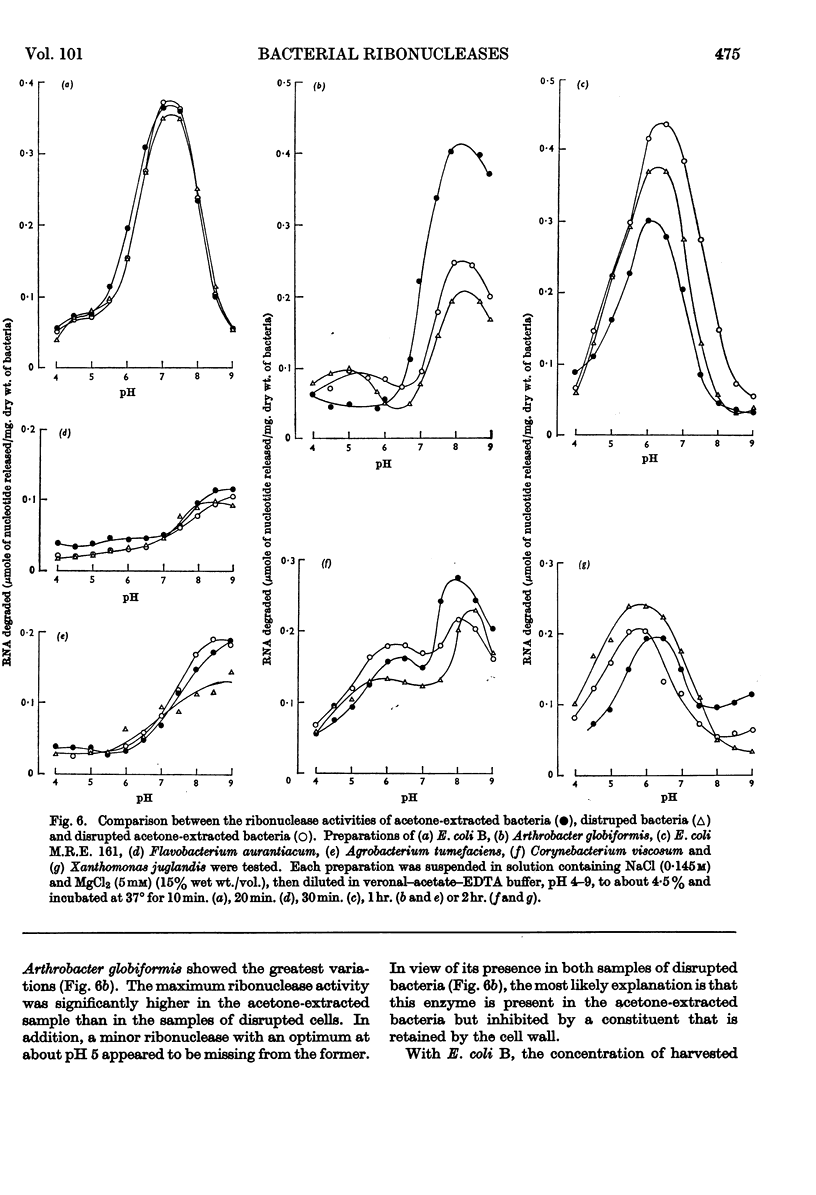
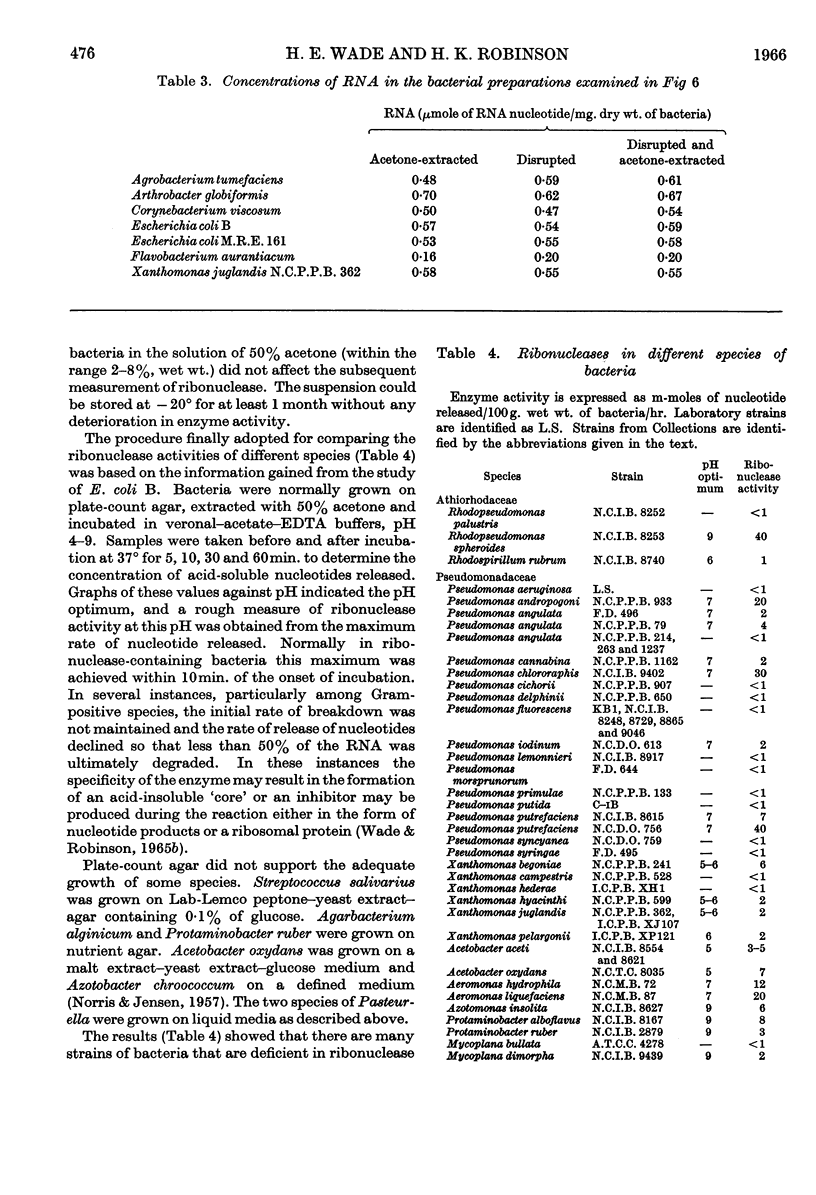
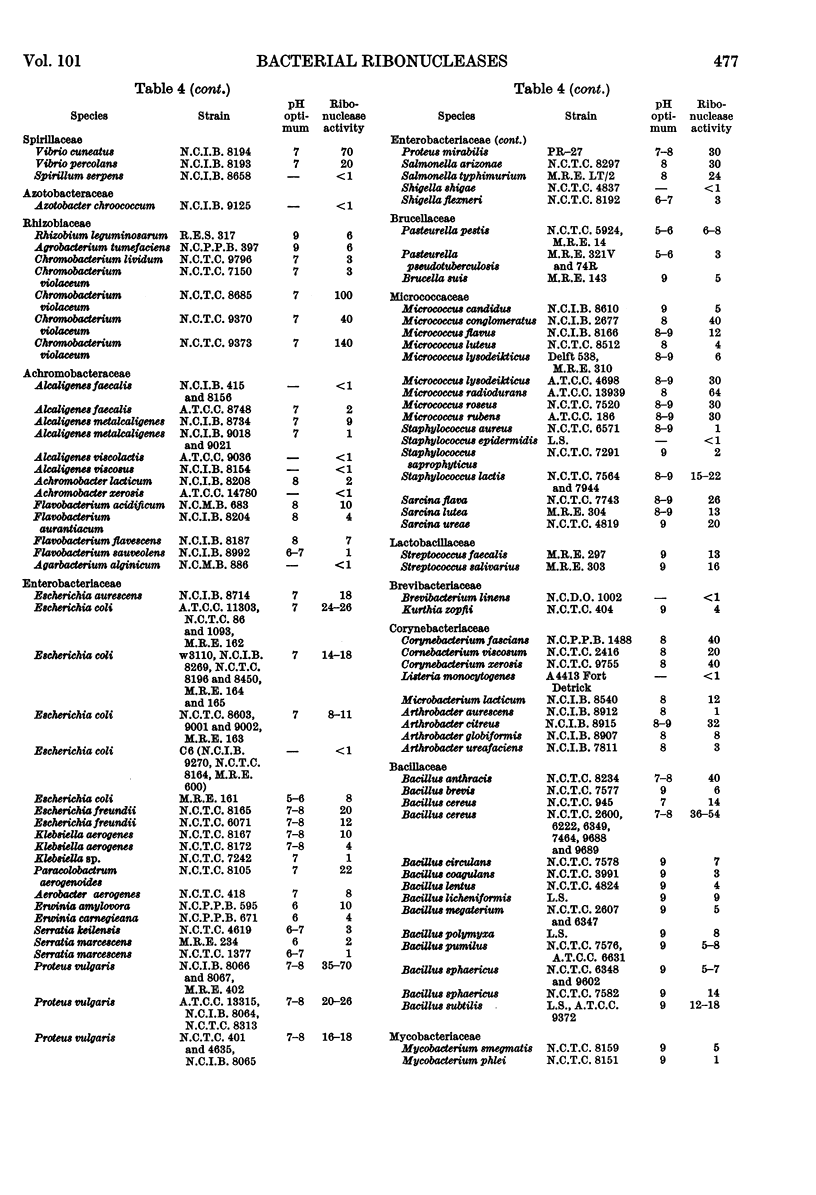
The distribution of ribonucleases among bacteria has been determined from the examination of a wide variety of species. Bacteria that had been growing rapidly on a solid medium were harvested, treated with acetone and incubated in the presence of EDTA between pH4 and pH9. The ribonuclease activity was determined from the rate at which acid-soluble nucleotides were released. Out of nearly 200 strains examined, about 30 did not contain a detectable ribonuclease. The pH optima of ribonucleases in the remainder were sufficiently distinctive to suggest a use in taxonomy. Escherichia coli B was examined in more detail to determine the factors responsible for variations in the ribonuclease content of this bacterium. Growth rate had little influence on ribonuclease content when a complex medium containing no readily assimilable carbohydrate was used; the addition of glucose resulted in a marked increase in ribonuclease and a dependence of enzyme content on growth rate. An increase in the concentration of sodium chloride in the medium decreased the ribonuclease content of bacteria growing on it.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANRAKU Y., MIZUNO D. A RIBONUCLEASE FROM THE DEBRIS OF ESCHERICHIA COLI. Biochem Biophys Res Commun. 1965 Feb 17;18:462–468. doi: 10.1016/0006-291x(65)90774-6. [DOI] [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. The basis of virulence in Pasteurella pestis: attempts to induce mutation from avirulence to virulence. Br J Exp Pathol. 1954 Apr;35(2):129–133. [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack K. A., Wade H. E. The sedimentation behaviour of ribonuclease-active and -inactive ribosomes from bacteria. Biochem J. 1965 Sep;96(3):671–680. doi: 10.1042/bj0960671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- NORRIS J. R., JENSEN H. L. Calcium requirements of Azotobacter. Nature. 1957 Dec 28;180(4600):1493–1494. doi: 10.1038/1801493a0. [DOI] [PubMed] [Google Scholar]
- ROTH J. S. Ribonuclease. III. Ribonuclease activity in rat liver and kidney. J Biol Chem. 1954 May;208(1):181–194. [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- SHEWAN J. M., HODGKISS W. A method for the rapid differentiation of certain nonpathogenic, asporogenous bacilli. Nature. 1954 Jan 30;173(4396):208–209. doi: 10.1038/173208b0. [DOI] [PubMed] [Google Scholar]
- SPAHR P. F. PURIFICATION AND PROPERTIES OF RIBONUCLEASE II FROM ESCHERICHIA COLI. J Biol Chem. 1964 Nov;239:3716–3726. [PubMed] [Google Scholar]
- SPIRIN A. S., KISELEV N. A., SHAKULOV R. S., BOGDANOV A. A. IZUCHENIE STRUKTURY RIBOSOM; OBRATIMOE RAZVORACHIVANIE RIBOSOMNYKH CHASTITS V RIBONUKLEOPROTEIDNYE TIAZHI I MODEL' UKLADKI. Biokhimiia. 1963 Sep-Oct;28:920–930. [PubMed] [Google Scholar]
- Tempest D. W., Hunter J. R. The influence of temperature and pH value on the macro-molecular composition of magnesium-limited and glycerol-limited Aerobacter aerogenes growing in a chemostat. J Gen Microbiol. 1965 Nov;41(2):267–273. doi: 10.1099/00221287-41-2-267. [DOI] [PubMed] [Google Scholar]
- WADE H. E., LOVETT S. Polynucleotide phosphorylase in ribosomes from Escherichia coli. Biochem J. 1961 Nov;81:319–328. doi: 10.1042/bj0810319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADE H. E., ROBINSON H. K. ABSENCE OF RIBONUCLEASE FROM THE RIBOSOMES OF PSEUDOMONAS FLUORESCENS. Nature. 1963 Nov 16;200:661–663. doi: 10.1038/200661a0. [DOI] [PubMed] [Google Scholar]
- WADE H. E. The autodegradation of ribonucleoprotein in Escherichia coli. Biochem J. 1961 Mar;78:457–472. doi: 10.1042/bj0780457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADE H. E. Variation in the phosphorus content of Escherichia coli during cultivation. J Gen Microbiol. 1952 Aug;7(1-2):24–30. doi: 10.1099/00221287-7-1-2-24. [DOI] [PubMed] [Google Scholar]
- Wade H. E., Robinson H. K. The distribution of ribosomal ribonucleic acids among subcellular fractions from bacteria and the adverse effect of the membrane fraction on the stability of ribosomes. Biochem J. 1965 Sep;96(3):753–765. doi: 10.1042/bj0960753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade H. E., Robinson H. K. The inhibition of ribosomal ribonuclease by bacterial ribosomes. Biochem J. 1965 Dec;97(3):747–753. doi: 10.1042/bj0970747. [DOI] [PMC free article] [PubMed] [Google Scholar]


