Abstract
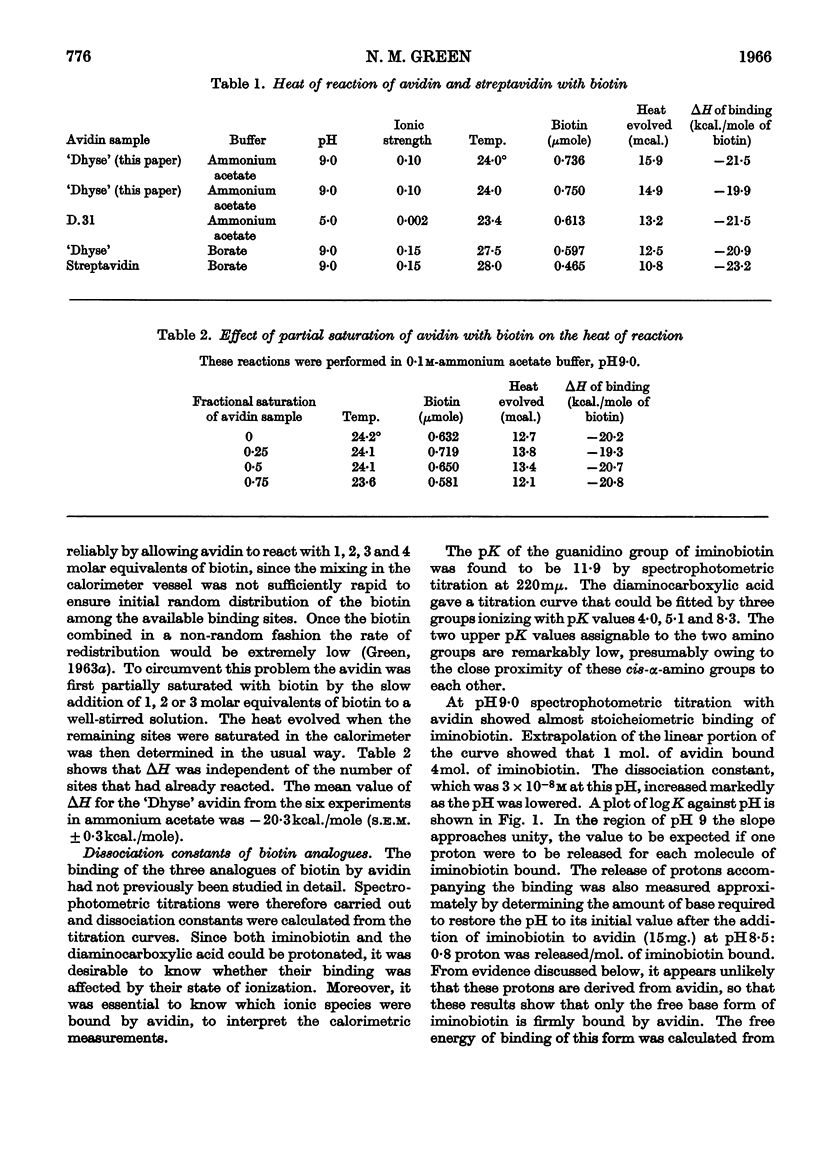
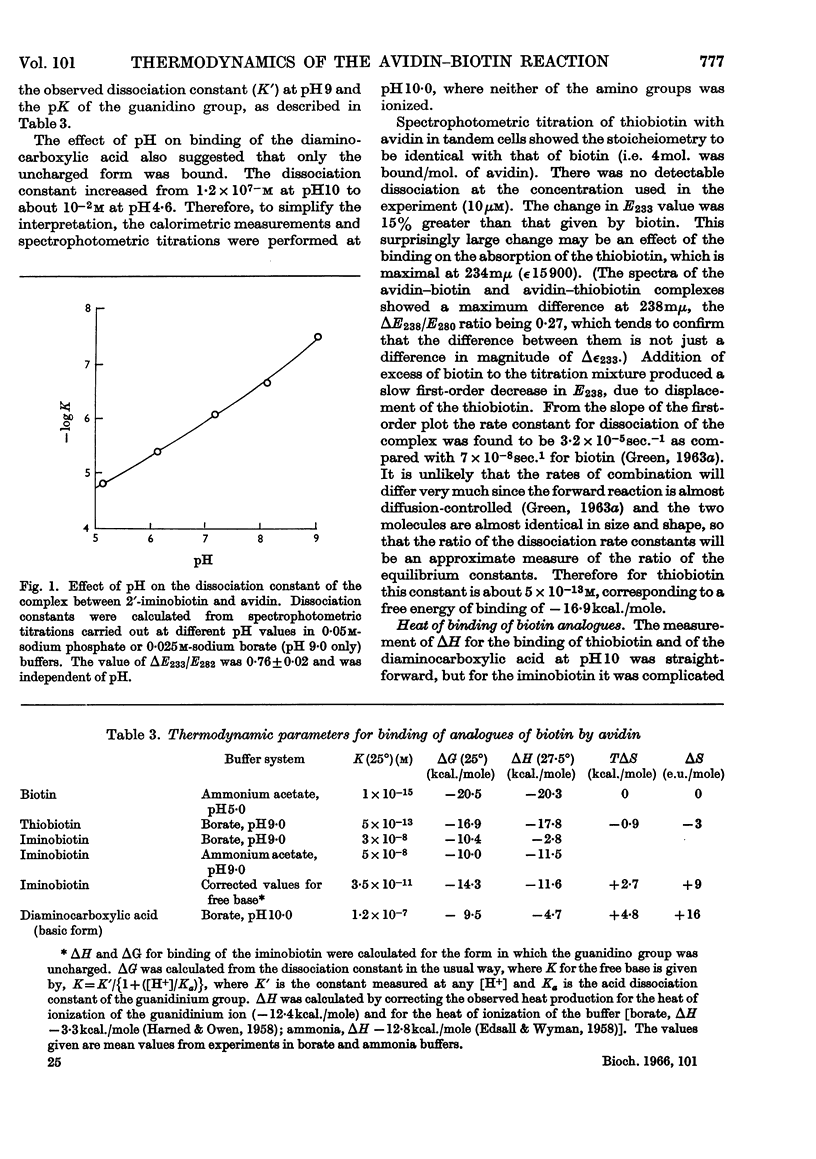
1. The reaction between avidin and biotin was found to be exothermic, ΔH being −20·3kcal./mole of biotin bound. The corresponding value of ΔH for streptavidin was −23kcal./mole. 2. The heat evolved was independent of the pH (between 5 and 9), of the buffer (borate or ammonia) and of the fractional saturation of the avidin with biotin. 3. The entropy change for the reaction was zero, and it is suggested that the entropy increase to be expected from hydrophobic interactions was counterbalanced by a decrease in entropy accompanying the formation of buried hydrogen bonds. 4. Modification of the potential hydrogen-bonding sites of the imidazolidone ring led to a decreased heat output and a positive entropy of reaction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANERJEE R. [Thermodynamic study of the heme-globin association. I. Dissociation equilibrium of metmyoglobin: thermodynamic data]. Biochim Biophys Acta. 1962 Oct 22;64:368–384. doi: 10.1016/0006-3002(62)90746-1. [DOI] [PubMed] [Google Scholar]
- CHAIET L., WOLF F. J. THE PROPERTIES OF STREPTAVIDIN, A BIOTIN-BINDING PROTEIN PRODUCED BY STREPTOMYCETES. Arch Biochem Biophys. 1964 Jul 20;106:1–5. doi: 10.1016/0003-9861(64)90150-x. [DOI] [PubMed] [Google Scholar]
- DHYSE F. G. A practical laboratory preparation of avidin concentrates for biological investigation. Proc Soc Exp Biol Med. 1954 Mar;85(3):515–517. doi: 10.3181/00379727-85-20936. [DOI] [PubMed] [Google Scholar]
- EISEN H. N., SISKIND G. W. VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry. 1964 Jul;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- GREEN N. M. AVIDIN. 1. THE USE OF (14-C)BIOTIN FOR KINETIC STUDIES AND FOR ASSAY. Biochem J. 1963 Dec;89:585–591. doi: 10.1042/bj0890585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN N. M. AVIDIN. 3. THE NATURE OF THE BIOTIN-BINDING SITE. Biochem J. 1963 Dec;89:599–609. doi: 10.1042/bj0890599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. M. Avidin. 5. Quenching of fluorescence by dinitrophenyl groups. Biochem J. 1964 Mar;90(3):564–568. doi: 10.1042/bj0900564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N. M., Melamed M. D. Optical rotatory dispersion, circular dichroism and far-ultraviolet spectra of avidin and streptavidin. Biochem J. 1966 Sep;100(3):614–621. doi: 10.1042/bj1000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFMANN K., AXELROD A. E. Microbiological activity of the guanido analogues of biotin and oxybiotin. J Biol Chem. 1950 Nov;187(1):29–33. [PubMed] [Google Scholar]
- KITZINGER C., BENZINGER T. H. Principle and method of heatburst microcalorimetry and the determination of free energy, enthalpy, and entropy changes. Methods Biochem Anal. 1960;8:309–360. doi: 10.1002/9780470110249.ch8. [DOI] [PubMed] [Google Scholar]
- MCMENAMY R. H. THE BINDING OF INDOLE ANALOGUES TO DEFATTED HUMAN SERUM ALBUMIN AT DIFFERENT CHLORIDE CONCENTRATIONS. J Biol Chem. 1964 Sep;239:2835–2841. [PubMed] [Google Scholar]
- MELAMED M. D., GREEN N. M. AVIDIN. 2. PURIFICATION AND COMPOSITION. Biochem J. 1963 Dec;89:591–599. doi: 10.1042/bj0890591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELAMED M. D., GREEN N. M. AVIDIN. 2. PURIFICATION AND COMPOSITION. Biochem J. 1963 Dec;89:591–599. doi: 10.1042/bj0890591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYER Y. P., SCHELLMAN J. A. The interaction of ribonuclease with purine and pyrimidine phosphates. I. Binding of adenosine 5'-monophosphate to ribonuclease. Biochim Biophys Acta. 1962 Mar 5;55:361–373. doi: 10.1016/0006-3002(62)90791-6. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Scruggs R. L. Heat of the reaction forming the three-stranded poly (A + 2U) complex. Biopolymers. 1965;3(4):491–496. doi: 10.1002/bip.1965.360030410. [DOI] [PubMed] [Google Scholar]
- Stryer L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J Mol Biol. 1965 Sep;13(2):482–495. doi: 10.1016/s0022-2836(65)80111-5. [DOI] [PubMed] [Google Scholar]


