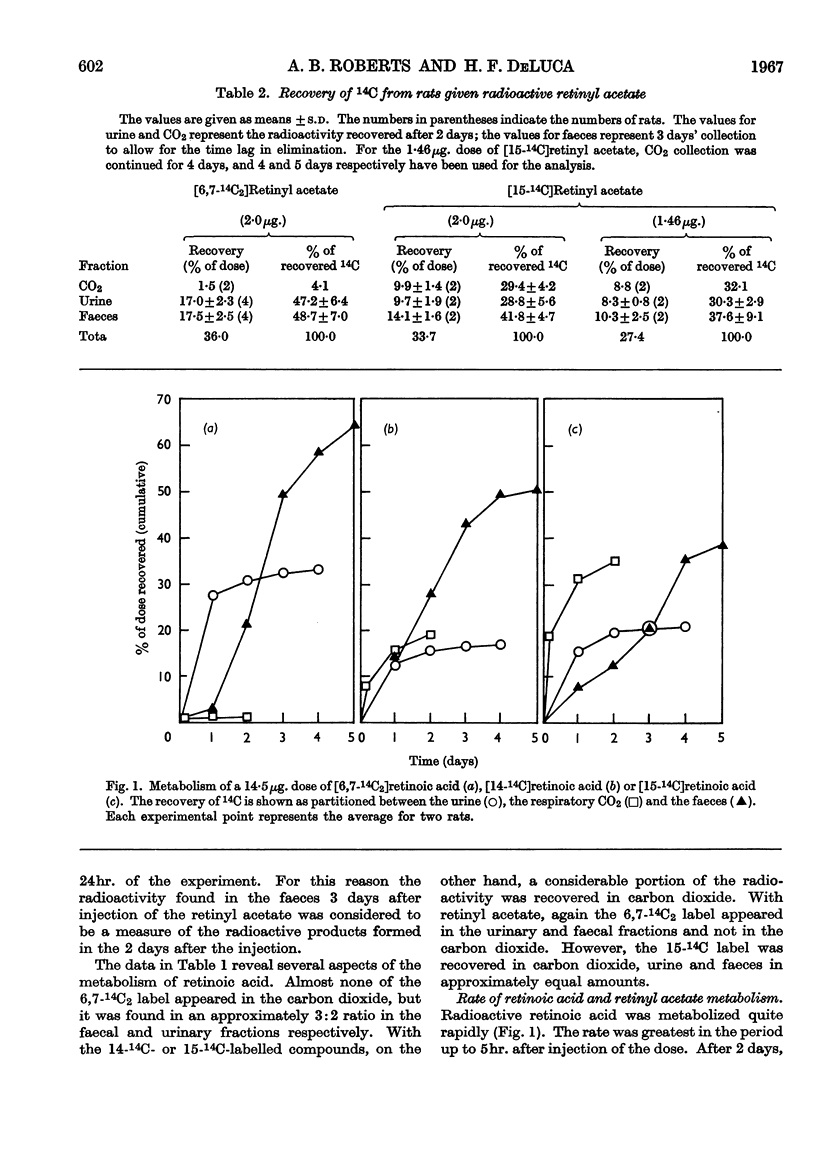
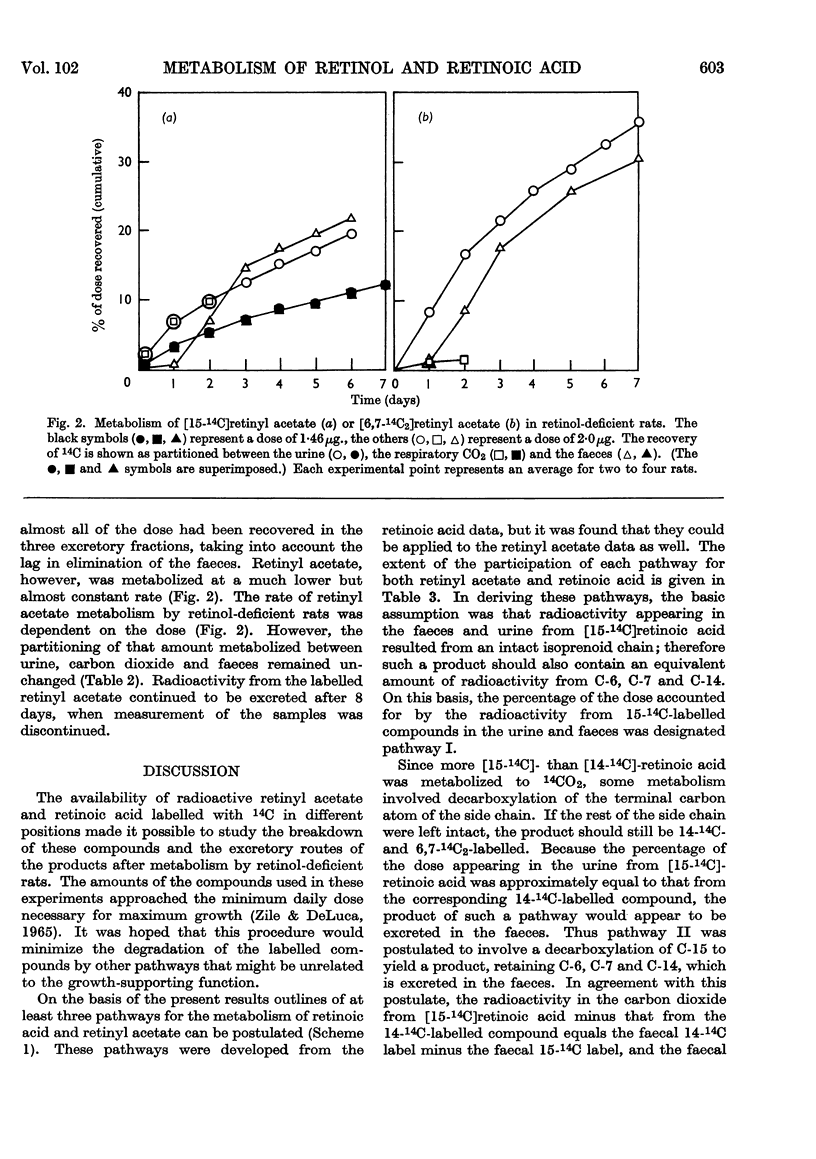
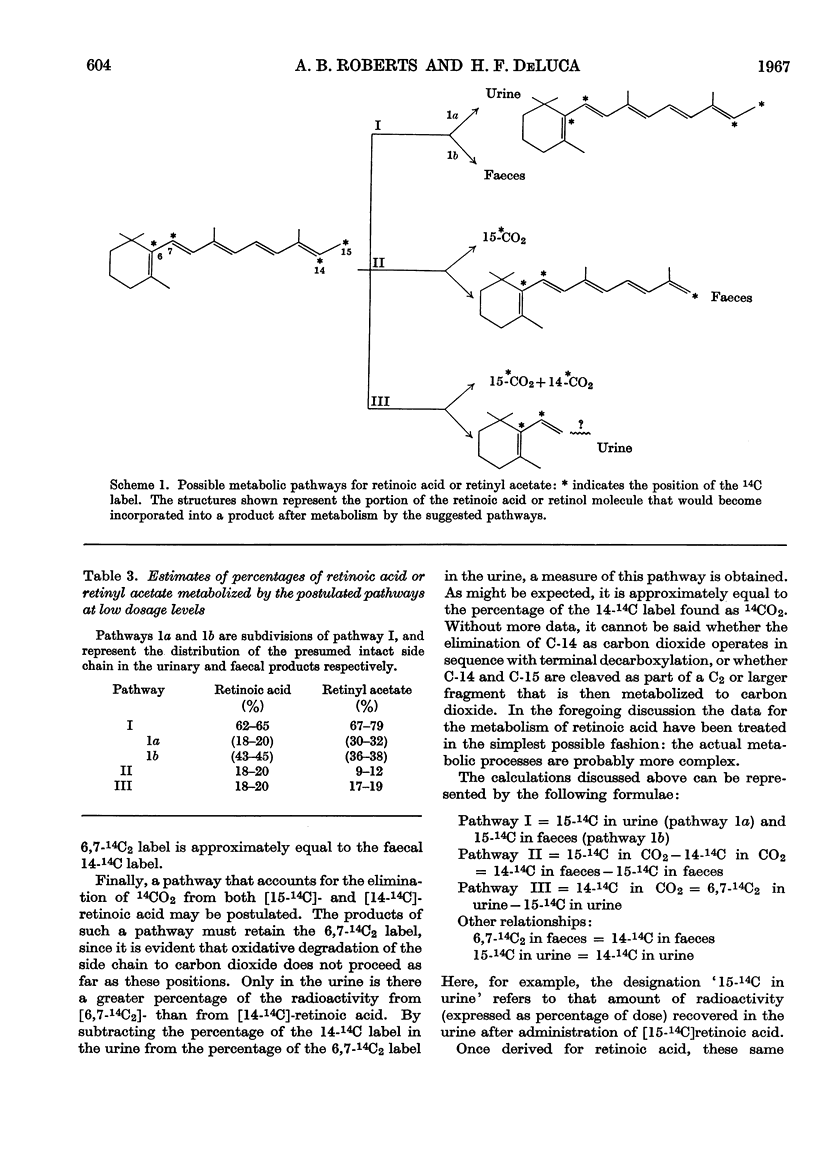
Abstract
1. The metabolism of retinoic acid and retinyl acetate labelled with 14C in various positions was studied after intravenous injection of physiological amounts of these compounds into retinol-deficient rats. 2. Analysis of the resultant radio-activity in the urine, carbon dioxide and faeces led to a postulation of the existence of three major pathways for the metabolism of these two compounds. 3. Evidence is presented that retinoic acid and retinol are metabolized by either the same or at least similar pathways and that retinol becomes oxidized to the carboxyl state before any degradation of the isoprenoid side chain occurs. 4. It is not possible to decide from these data whether retinoic acid is an intermediate in the retinol pathway. 5. Possible sites for the regulation of retinol metabolism are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DELUCA H. F., MANATT M. R., MADSEN N., OLSON E. B. ACTION OF VITAMIN A ON LIVER HOMOGENATE OXIDATION OF TRICARBOXYLIC ACID CYCLE INTERMEDIATES. J Nutr. 1963 Dec;81:383–386. doi: 10.1093/jn/81.4.383. [DOI] [PubMed] [Google Scholar]
- DUNAGIN P. E., Jr, MEADOWS E. H., Jr, OLSON J. A. RETINOYL BETA-GLUCURONIC ACID: A MAJOR METABOLITE OF VITAMIN A IN RAT BILE. Science. 1965 Apr 2;148(3666):86–87. doi: 10.1126/science.148.3666.86. [DOI] [PubMed] [Google Scholar]
- DUNAGIN P. E., Jr, ZACHMAN R. D., OLSON J. A. IDENTIFICATION OF FREE AND CONJUGATED RETINOIC ACID AS A PRODUCT OF RETINAL (VITAMIN A ALDEHYDE) METABOLISM IN THE RAT IN VIVO. Biochim Biophys Acta. 1964 Aug 19;90:432–434. doi: 10.1016/0304-4165(64)90218-1. [DOI] [PubMed] [Google Scholar]
- Deshmukh D. S., Malathi P., Ganguly J. Rapid conversion of retinal (vitamin A aldehyde) to retinoic acid (vitamin A acid) in the living rat. Biochim Biophys Acta. 1965 Aug 24;107(1):120–122. doi: 10.1016/0304-4165(65)90394-6. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Wald G. THE BIOLOGICAL FUNCTION OF VITAMIN A ACID. Proc Natl Acad Sci U S A. 1960 May;46(5):587–608. doi: 10.1073/pnas.46.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUTTERMAN S. Enzymatic oxidation of vitamin A aldehyde to vitamin A acid. J Biol Chem. 1962 Mar;237:677–680. [PubMed] [Google Scholar]
- FUTTERMAN S., SASLAW L. D. The estimation of vitamin A aldehyde with thiobabituric acid. J Biol Chem. 1961 Jun;236:1652–1657. [PubMed] [Google Scholar]
- KELLY R. G., PEETS E. A., GORDON S., BUYSKE D. A. Determination of C-14 and H3 in biological samples by Schoeniger combustion and liquid scintillation techniques. Anal Biochem. 1961 Jun;2:267–273. doi: 10.1016/s0003-2697(61)80010-9. [DOI] [PubMed] [Google Scholar]
- KRISHNAMURTHY S., BIERI J. G., ANDREWS E. L. Metabolism and biological activity of vitamin A acid in the chick. J Nutr. 1963 Apr;79:503–510. doi: 10.1093/jn/79.4.503. [DOI] [PubMed] [Google Scholar]
- Mahadevan S., Murthy S. K., Ganguly J. Enzymic oxidation of vitamin A aldehyde to vitamin A acid by rat liver. Biochem J. 1962 Nov;85(2):326–331. doi: 10.1042/bj0850326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZACHMAN R. D., OLSON J. A. A comparison of retinene reductase and alcohol dehydrogenase of rat liver. J Biol Chem. 1961 Aug;236:2309–2313. [PubMed] [Google Scholar]
- Zachman R. D., Dunagin P. E., Jr, Olson J. A. Formation and enterohepatic circulation of metabolites of retinol and retinoic acid in bile duct-cannulated rats. J Lipid Res. 1966 Jan;7(1):3–9. [PubMed] [Google Scholar]
- Zile M., Deluca H. F. A biologically active metabolite of retinoic acid from rat liver. Biochem J. 1965 Oct;97(1):180–186. doi: 10.1042/bj0970180. [DOI] [PMC free article] [PubMed] [Google Scholar]


