Abstract
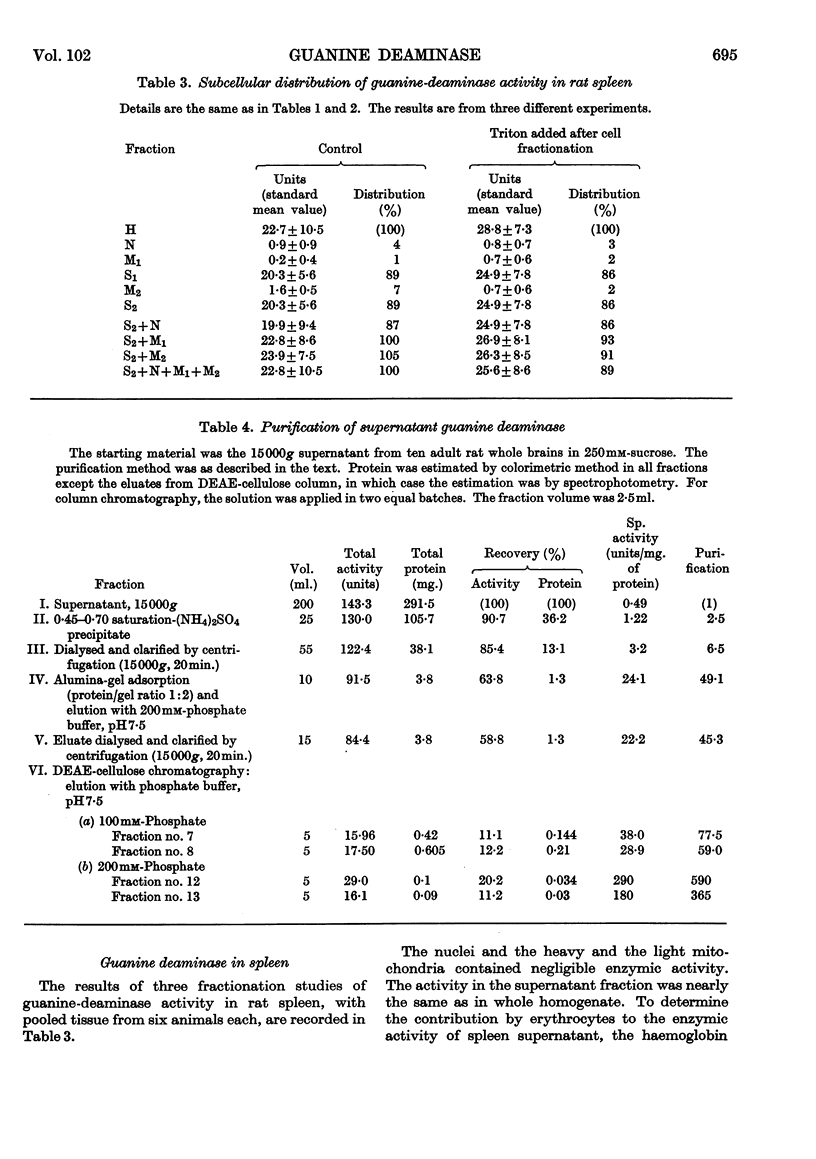
1. In kidney, but not in rat whole brain and liver, guanine-deaminase activity was localized almost exclusively in the 15000g supernatant fraction of iso-osmotic sucrose homogenates. However, as in brain and liver, the enzymic activity recovered in the supernatant was higher than that in the whole homogenate. The particulate fractions of kidney, especially the heavy mitochondria, brought about powerful inhibition of the supernatant guanine-deaminase activity. 2. In spleen, as in kidney, guanine-deaminase activity was localized in the 15000g supernatant fraction of iso-osmotic sucrose homogenates. However, the particulate fractions did not inhibit the activity of the supernatant. 3. Guanine-deaminase activity in rat brain was absent from the cerebellum and present only in the cerebral hemispheres. The inhibitor of guanine deaminase was located exclusively in the cerebellum, where it was associated with the particles sedimenting at 5000g from sucrose homogenates. 4. Homogenates of cerebral hemispheres, the separated cortex or the remaining portion of the hemispheres had significantly higher guanine-deaminase activity than homogenates of whole brain. The enzymic activity of the subcellular particulate fractions was nearly the same. 5. Guanine deaminase was purified from the 15000g supernatant of sucrose homogenates of whole brain. The enzyme separated as two distinct fractions, A and B, on DEAE-cellulose columns. 6. The guanine-deaminase activity of the light-mitochondrial fraction of whole brain was fully exposed and solubilized by treatment with Triton X-100, and partially purified. 7. Tested in the form of crude preparations, the inhibitor from kidney did not act on the brain and liver supernatant enzymes and the inhibitor from cerebellum did not act on kidney enzyme, but the inhibitor from liver acted on both brain and kidney enzyme. 8. The inhibitor of guanine deaminase was purified from the heavy mitochondria of whole brain and liver and the 5000g residue of cerebellum, isolated from iso-osmotic homogenates. The inhibitor appeared to be protein in nature and was heat-labile. The inhibition of the enzyme was non-competitive. 9. Kinetic, immunochemical and electrophoretic studies with the preparations purified from brain revealed that the enzyme from light mitochondria was distinct from enzyme B from the supernatant. A distinction between the two forms of supernatant enzyme was less certain. 10. Guanine deaminase isolated from light mitochondria of brain did not react with 8-azaguanine or with the inhibitor isolated from heavy mitochondria.
Full text
PDF









Selected References
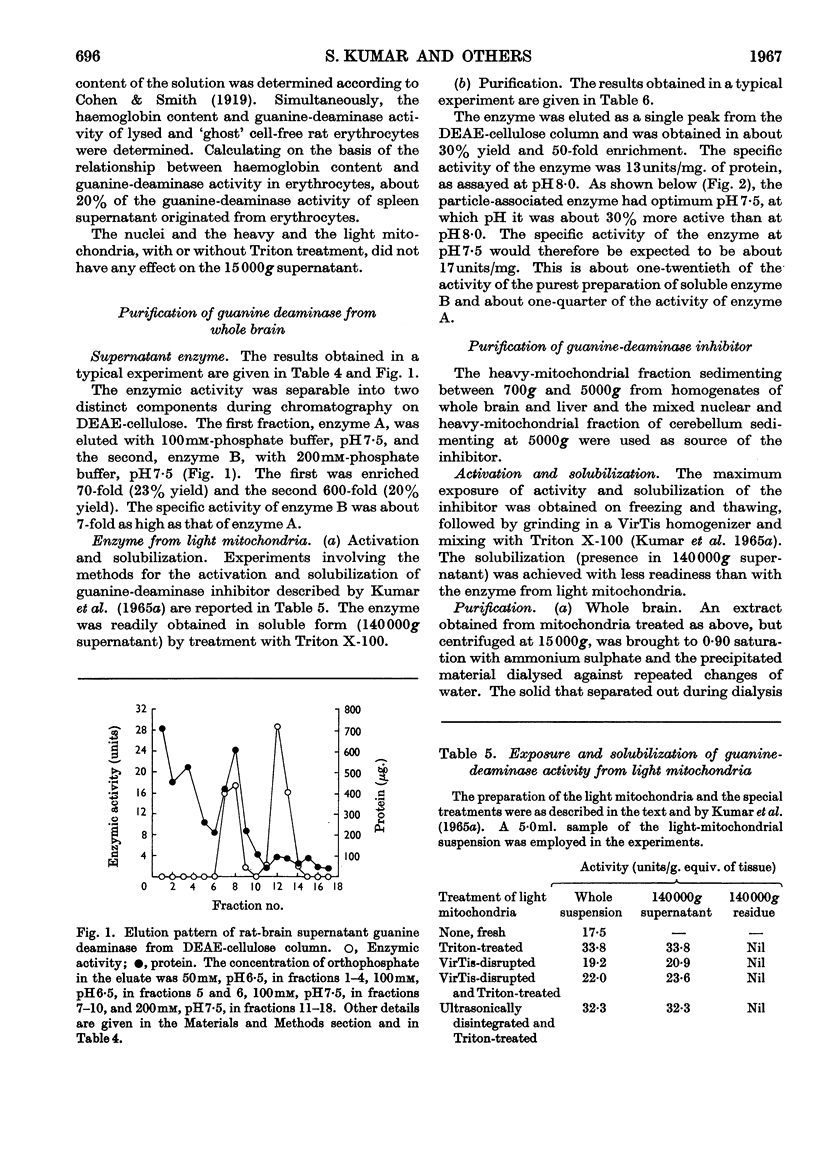
These references are in PubMed. This may not be the complete list of references from this article.
- Conway E. J. An absorption apparatus for the micro-determination of certain volatile substances: The determination of urea and ammonia in body fluids. Biochem J. 1933;27(2):430–434. [PMC free article] [PubMed] [Google Scholar]
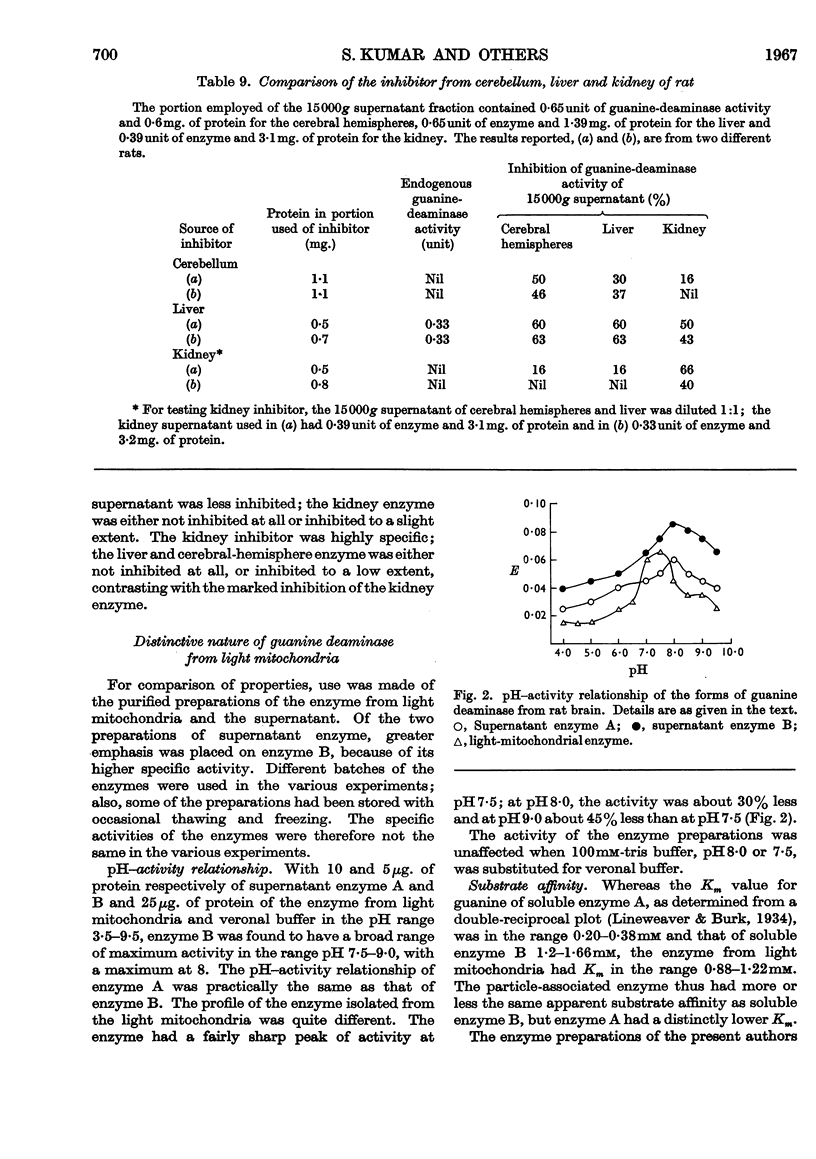
- GRIMM F. C., DOHERTY D. G. Properties of the two forms of malic dehydrogenase from beef heart. J Biol Chem. 1961 Jul;236:1980–1985. [PubMed] [Google Scholar]
- HIRSCHBERG E., KREAM J., GELLHORN A. Enzymatic deamination of 8-azaguanine in normal and neoplastic tissues. Cancer Res. 1952 Jul;12(7):524–527. [PubMed] [Google Scholar]
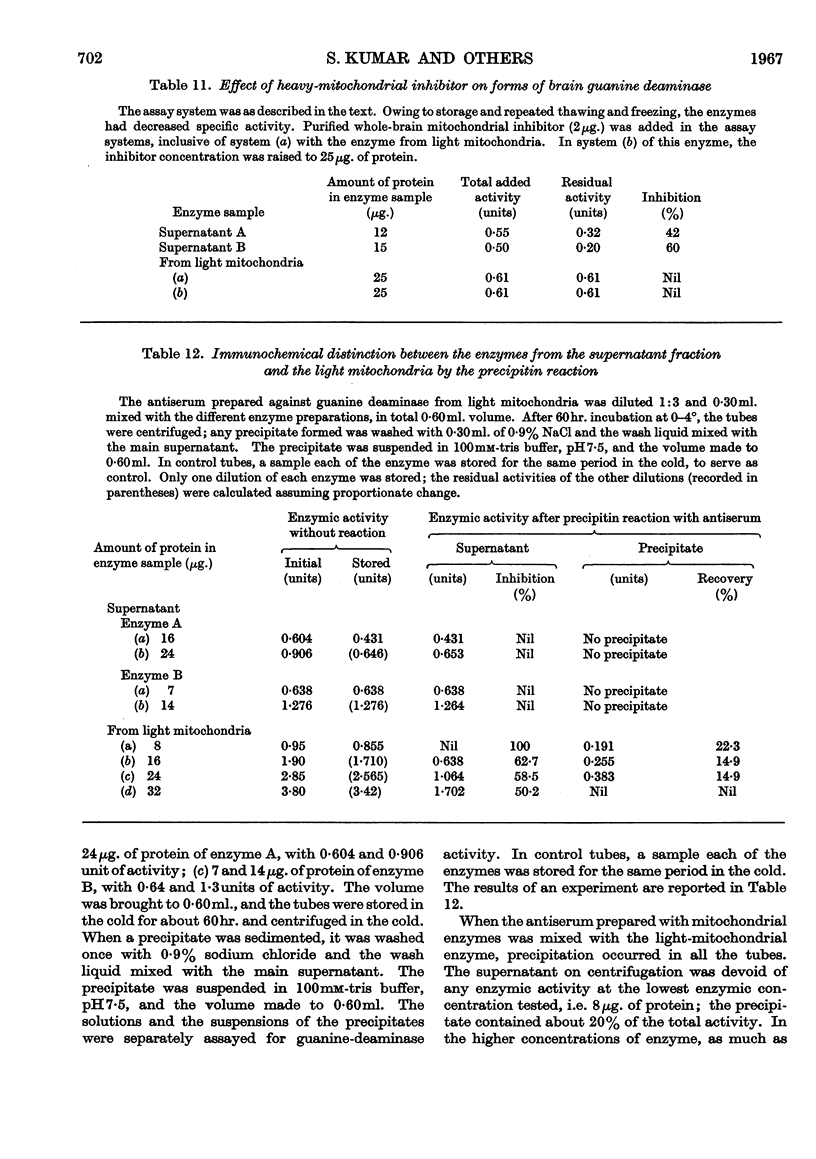
- KUMAR S., TEWARI K. K., KRISHNAN P. S. GUANINE-DEAMINASE ACTIVITY IN RAT BRAIN AND LIVER. Biochem J. 1965 Jun;95:797–802. doi: 10.1042/bj0950797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitto G. B., Wassarman P. M., Michjeda J., Kaplan N. O. Multiple forms of mitochondrial malate dehydrogenases. Biochem Biophys Res Commun. 1966 Jan 4;22(1):75–81. doi: 10.1016/0006-291x(66)90605-x. [DOI] [PubMed] [Google Scholar]
- Kumar S., Tewari K. K., Krishnan P. S. Partial purification of guanine deaminase inhibitor from rat brain. J Neurochem. 1965 Dec;12(12):1003–1004. doi: 10.1111/j.1471-4159.1965.tb10260.x. [DOI] [PubMed] [Google Scholar]
- LEVINE R., HALL T. C., HARRIS C. A. Guanase activity in normal and neoplastic human tissue. Cancer. 1963 Feb;16:269–272. doi: 10.1002/1097-0142(196302)16:2<269::aid-cncr2820160218>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANSOOR M., KALYANKAR G. D., TALWAR G. P. BRAIN GUANINE DEAMINASE: PURIFICATION, PROPERTIES AND REGIONAL DISTRIBUTION. Biochim Biophys Acta. 1963 Oct 1;77:307–317. doi: 10.1016/0006-3002(63)90501-8. [DOI] [PubMed] [Google Scholar]
- ROUSH A., NORRIS E. R. Deamination of 8-azaguanine by guanase. Arch Biochem. 1950 Nov;29(1):124–129. [PubMed] [Google Scholar]
- THORNE C. J. Characterisation of two malic dehydrogenases from rat liver. Biochim Biophys Acta. 1960 Jul 29;42:175–176. doi: 10.1016/0006-3002(60)90773-3. [DOI] [PubMed] [Google Scholar]


