Abstract
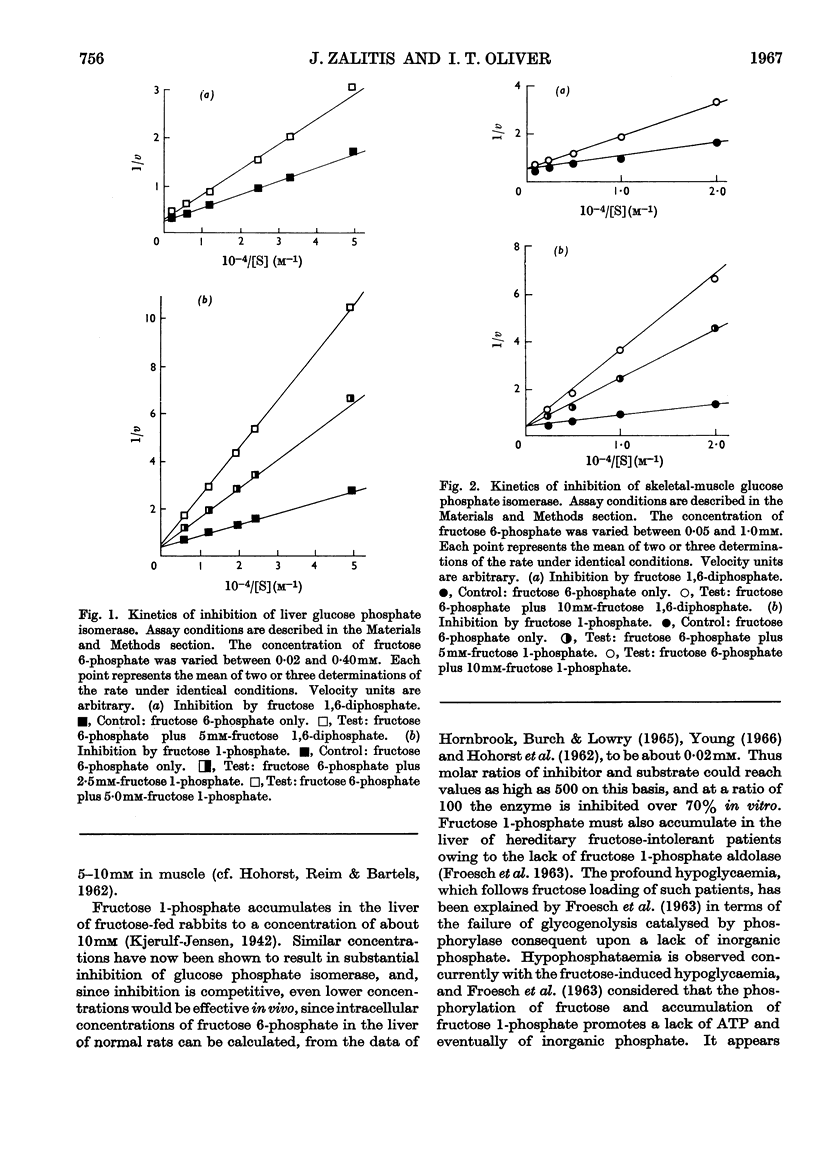
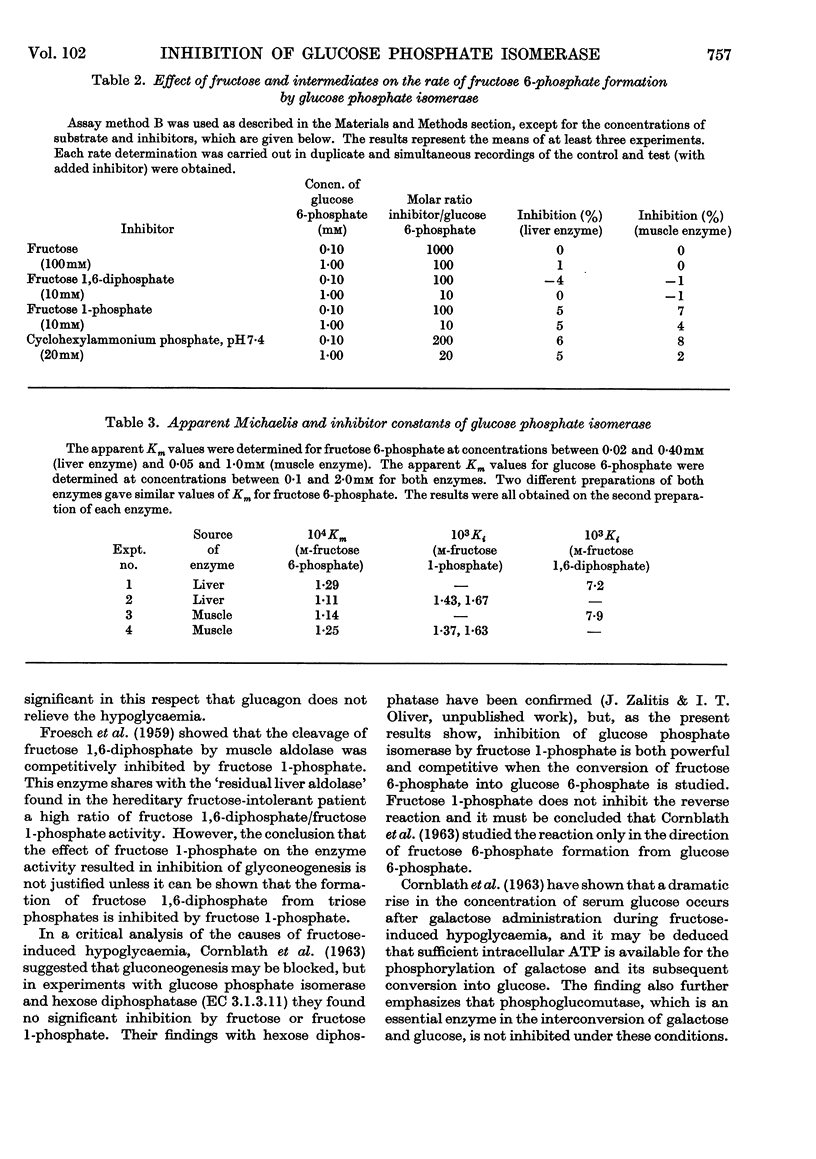
1. Purified rabbit-muscle and -liver glucose phosphate isomerase, free of contaminating enzyme activities that could interfere with the assay procedures, were tested for inhibition by fructose, fructose 1-phosphate and fructose 1,6-diphosphate. 2. Fructose 1-phosphate and fructose 1,6-diphosphate are both competitive with fructose 6-phosphate in the enzymic reaction, the apparent Ki values being 1·37×10−3−1·67×10−3m for fructose 1-phosphate and 7·2×10−3−7·9×10−3m for fructose 1,6-diphosphate; fructose and inorganic phosphate were without effect. 3. The apparent Km values for both liver and muscle enzymes at pH7·4 and 30° were 1·11×10−4−1·29×10−4m for fructose 6-phosphate, determined under the conditions in this paper. 4. In the reverse reaction, fructose, fructose 1-phosphate and fructose 1,6-diphosphate did not significantly inhibit the conversion of glucose 6-phosphate into fructose 6-phosphate. 5. The apparent Km values for glucose 6-phosphate were in the range 5·6×10−4−8·5×10−4m. 6. The competitive inhibition of hepatic glucose phosphate isomerase by fructose 1-phosphate is discussed in relation to the mechanism of fructose-induced hypoglycaemia in hereditary fructose intolerance.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J., Oliver I. T. Ketohexokinase, isoenzymes of glucokinase and glycogen synthesis from hexoses in neonatal rat liver. Biochem J. 1964 Feb;90(2):261–268. doi: 10.1042/bj0900261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORNBLATH M., ROSENTHAL I. M., REISNER S. H., WYBREGT S. H., CRANE R. K. HEREDITARY FRUCTOSE INTOLERANCE. N Engl J Med. 1963 Dec 12;269:1271–1278. doi: 10.1056/NEJM196312122692401. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A., CRANE R. K. THE INFLUENCE OF THE METHOD OF ASSAY ON THE APPARENT SPECIFICITY OF RABBIT-LIVER ALDOLASE. Biochim Biophys Acta. 1964 Apr 6;85:132–140. doi: 10.1016/0926-6569(64)90173-7. [DOI] [PubMed] [Google Scholar]
- DIEDRICH D. F., ANDERSON L. Separation of galactose I-phosphate from other hexose phosphates by ion exchange. Anal Biochem. 1961 Feb;2:68–79. doi: 10.1016/0003-2697(61)90041-0. [DOI] [PubMed] [Google Scholar]
- DUBOIS R., LOEB H., OOMS H. A., GILLET P., BARTMAN J., CHAMPENOIS A. [Study of a case of functional hypoglycemia caused by intolerance to fructose]. Helv Paediatr Acta. 1961 Apr;16:90–96. [PubMed] [Google Scholar]
- HOHORST H. J., REIM M., BARTELS H. Equilibria of two-partner reactions of energy supplying metabolism in muscle. Biochem Biophys Res Commun. 1962 Apr 3;7:137–141. doi: 10.1016/0006-291x(62)90162-6. [DOI] [PubMed] [Google Scholar]
- HORNBROOK K. R., BURCH H. B., LOWRY O. H. CHANGES IN SUBSTRATE LEVELS IN LIVER DURING GLYCOGEN SYNTHESIS INDUCED BY LACTATE AND HYDROCORTISONE. Biochem Biophys Res Commun. 1965 Jan 18;18:206–211. doi: 10.1016/0006-291x(65)90741-2. [DOI] [PubMed] [Google Scholar]
- KAHANA S. E., LOWRY O. H., SCHULZ D. W., PASSONNEAU J. V., CRAWFORD E. J. The kinetics of phosphoglucoisomerase. J Biol Chem. 1960 Aug;235:2178–2184. [PubMed] [Google Scholar]
- KLENOW H. On the nature of the salt inhibition of some reactions catalyzed by phosphoglucomutase preparations. Arch Biochem Biophys. 1955 Oct;58(2):288–297. doi: 10.1016/0003-9861(55)90130-2. [DOI] [PubMed] [Google Scholar]
- KREBS H. THE CROONIAN LECTURE, 1963. GLUCONEOGENESIS. Proc R Soc Lond B Biol Sci. 1964 Mar 17;159:545–564. doi: 10.1098/rspb.1964.0019. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NOLTMANN E. A. ISOLATION OF CRYSTALLINE PHOSPHOGLUCOSE ISOMERASE FROM RABBIT MUSCLE. J Biol Chem. 1964 May;239:1545–1550. [PubMed] [Google Scholar]
- PARR C. W. Inhibition of phosphoglucose isomerase. Nature. 1956 Dec 22;178(4547):1401–1401. doi: 10.1038/1781401a0. [DOI] [PubMed] [Google Scholar]
- POGELL B. M., McGILVERY R. W. Partial purification of fructose-1,6-diphosphatase. J Biol Chem. 1954 May;208(1):149–157. [PubMed] [Google Scholar]
- RAJAGOPAL M. V., SOHONIE K. Studies on the sea anemone Gyrostoma sp; lipids of Gyrostoma sp. Biochem J. 1957 Jan;65(1):34–36. doi: 10.1042/bj0650034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHULL K. H., ASHMORE J., MAYER J. Hexokinase, glucose-6-phosphatase and phosphorylase levels in hereditarily obese-hyperglycemic mice. Arch Biochem Biophys. 1956 May;62(1):210–216. doi: 10.1016/0003-9861(56)90104-7. [DOI] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. SOME PROPERTIES OF FRUCTOSE 1,6-DIPHOSPHATASE OF RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLUCONEOGENESIS. Biochem J. 1965 Jun;95:767–774. doi: 10.1042/bj0950767. [DOI] [PMC free article] [PubMed] [Google Scholar]


