Abstract
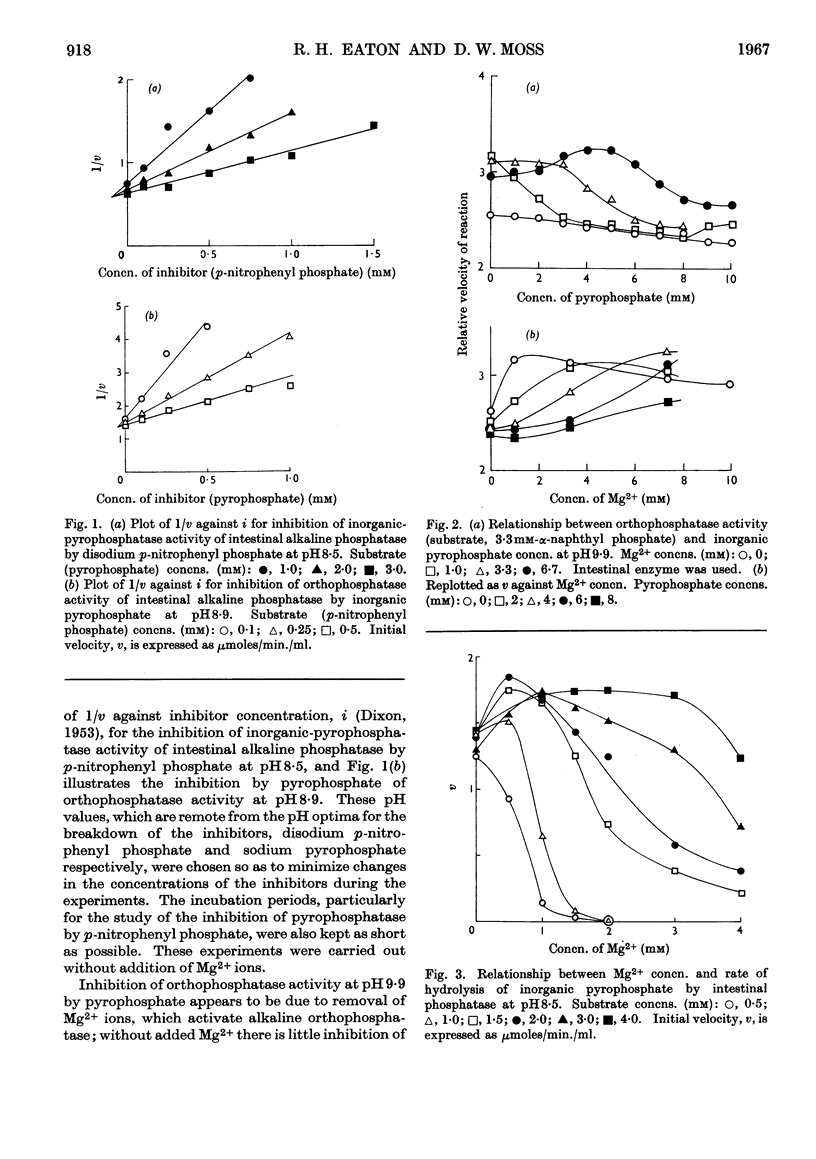
1. Inhibition of the pyrophosphatase and orthophosphatase activities of human liver and small-intestinal alkaline-phosphatase preparations by different classes of inhibitors has been studied. 2. Each type of substrate, pyrophosphate or orthophosphate, is a competitive inhibitor of hydrolysis of the other type. 3. l-Phenylalanine is a non-competitive inhibitor of both types of activity of the intestinal preparation, but inhibits neither activity of the liver enzyme. Arsenate is a competitive inhibitor of both activities of both preparations. For a given inhibitor, the values of Ki are independent of the type of substrate used when measurements are made at the same pH. 4. Mg2+ ions activate orthophosphatase but inhibit pyrophosphatase, except in very low concentrations. 5. These results are compatible with the presence in each tissue preparation of a single enzyme with one type of active centre, possessing both orthophosphatase and pyrophosphatase activities.
Full text
PDF
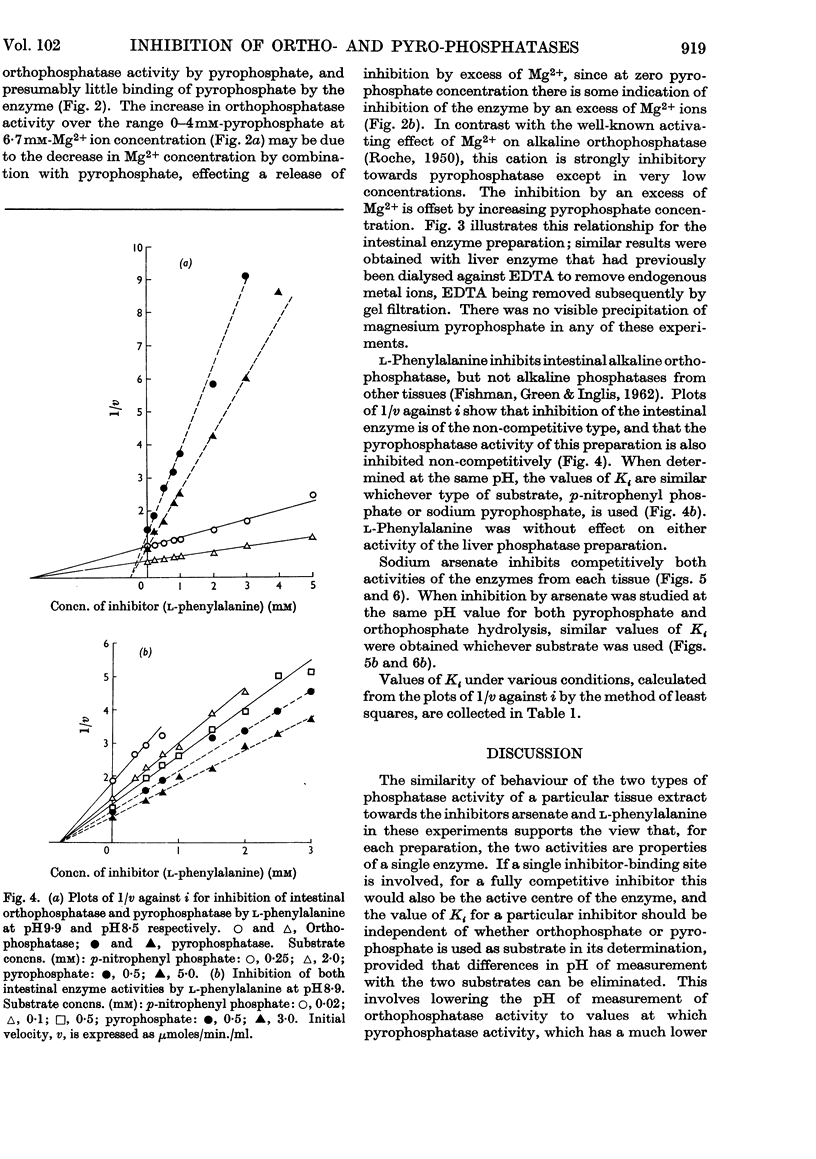
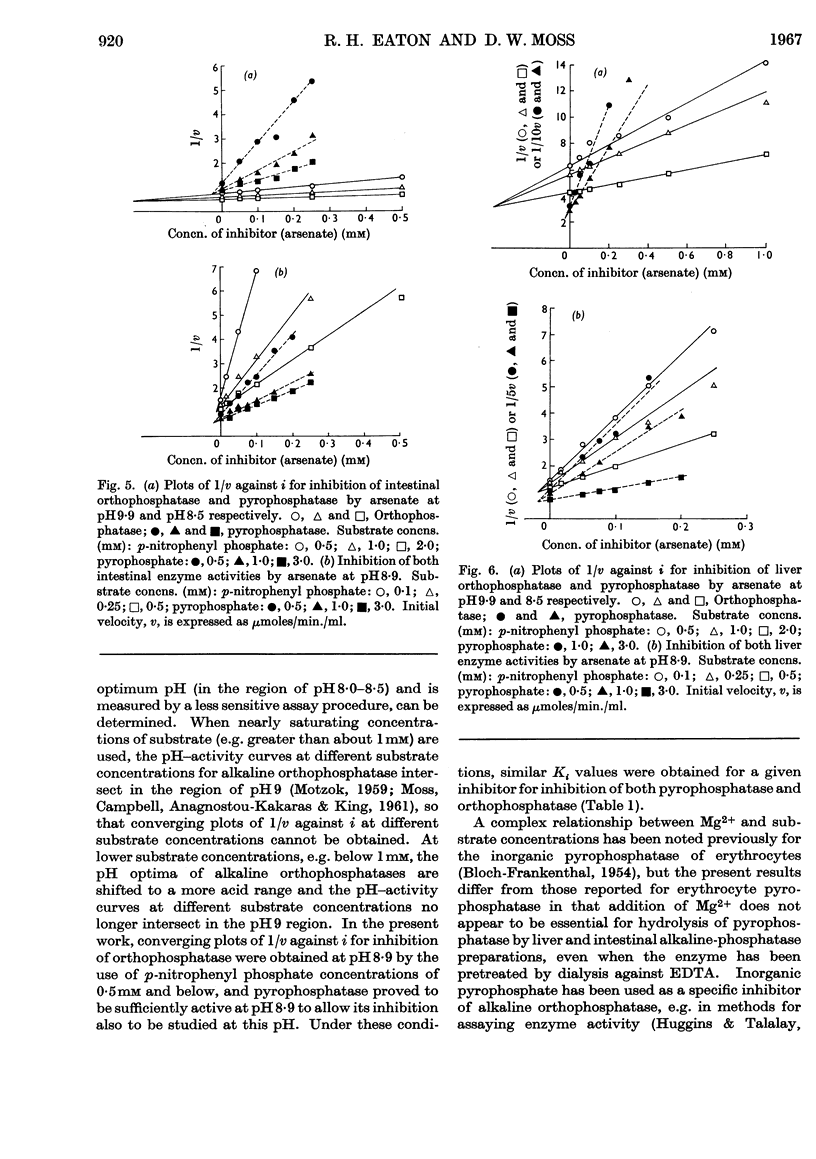

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOCH-FRANKENTHAL L. The role of magnesium in the hydrolysis of sodium pyrophosphate by inorganic pyrophosphatase. Biochem J. 1954 May;57(1):87–92. doi: 10.1042/bj0570087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELSAL J. L., MANHOURI H. Etude comparative des dosages colorimétriques du phosphore. IV. Dosage de l'orthophosphate en présence d'esters phosphoriques; nouvelles méthodes. Bull Soc Chim Biol (Paris) 1958;40(11):1623–1636. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delory G. E. A sodium carbonate-bicarbonate buffer for alkaline phosphatases. Biochem J. 1945;39(3):245–245. [PMC free article] [PubMed] [Google Scholar]
- FISHMAN W. H., GREEN S., INGLIS N. I. Organ-specific behavior exhibited by rat intestine and liver alkaline phosphatase. Biochim Biophys Acta. 1962 Aug 13;62:363–375. doi: 10.1016/0006-3002(62)90266-4. [DOI] [PubMed] [Google Scholar]
- MOSS D. W., CAMPBELL D. M., ANAGNOSTOU-KAKARAS E., KING E. J. Characterization of tissue alkaline phosphatases and their partial purification by starch-gel electrophoresis. Biochem J. 1961 Nov;81:441–447. doi: 10.1042/bj0810441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTZOK I. Studies on alkaline phosphatases. I. Kinetics of plasma phosphatase of normal and rachitic chicks. Biochem J. 1959 May;72(1):169–177. doi: 10.1042/bj0720169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. W. A note on the spectrophotometric estimation of alkaline phosphatase activity. Enzymologia. 1966 Oct 31;31(4):193–202. [PubMed] [Google Scholar]
- Moss D. W., Eaton R. H., Smith J. K., Whitby L. G. Association of inorganic-pyrophosphatase activity with human alkaline-phosphatase preparations. Biochem J. 1967 Jan;102(1):53–57. doi: 10.1042/bj1020053. [DOI] [PMC free article] [PubMed] [Google Scholar]


