Abstract
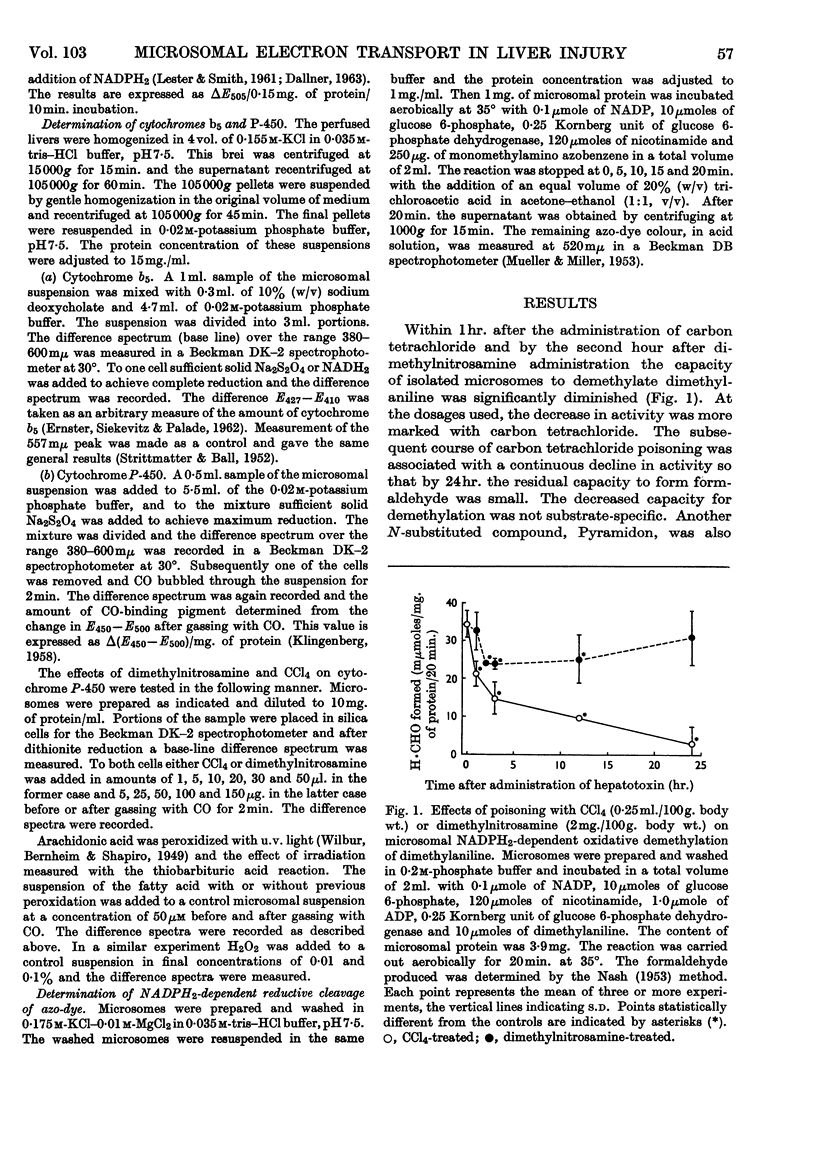
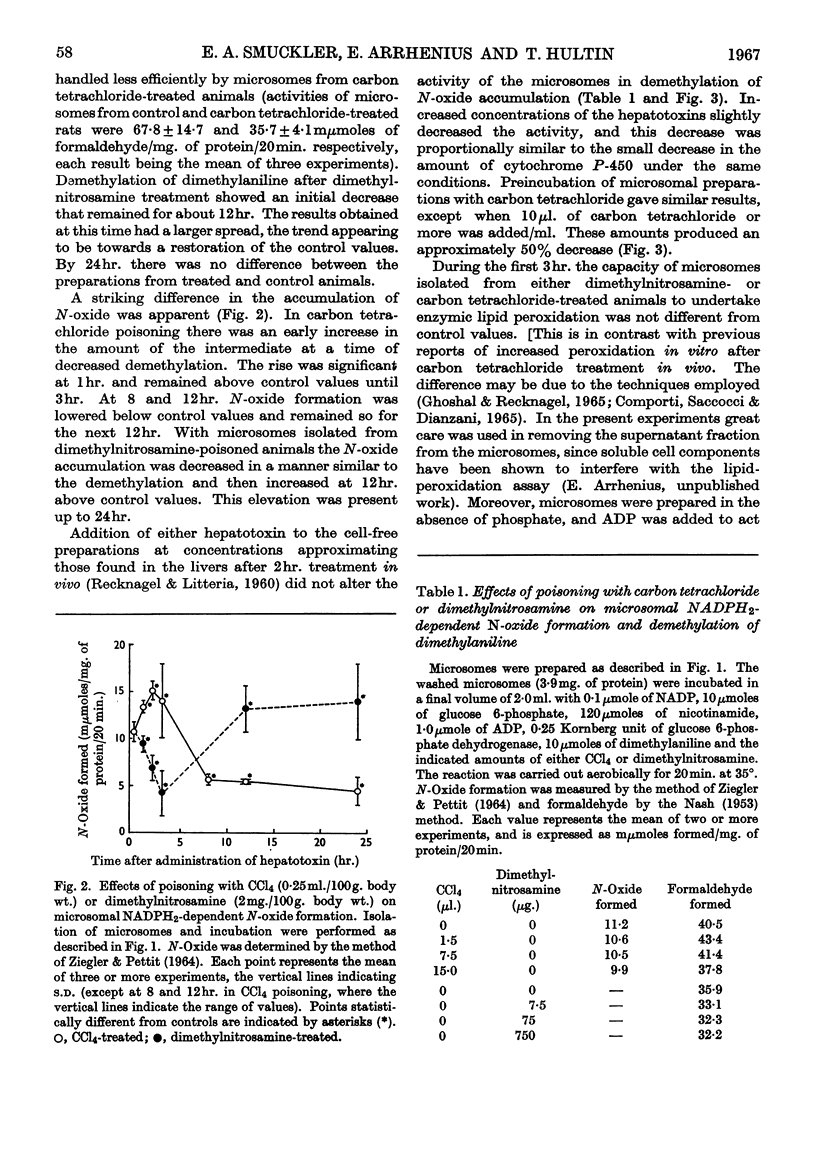
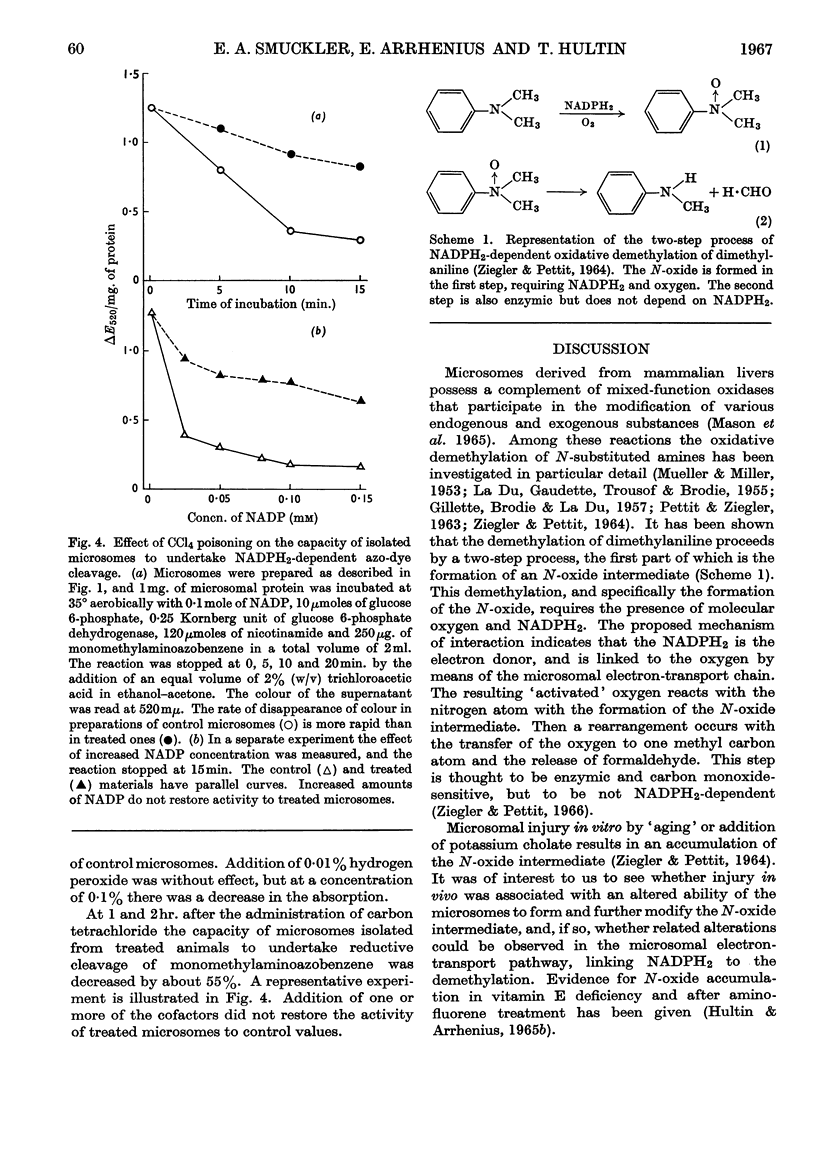
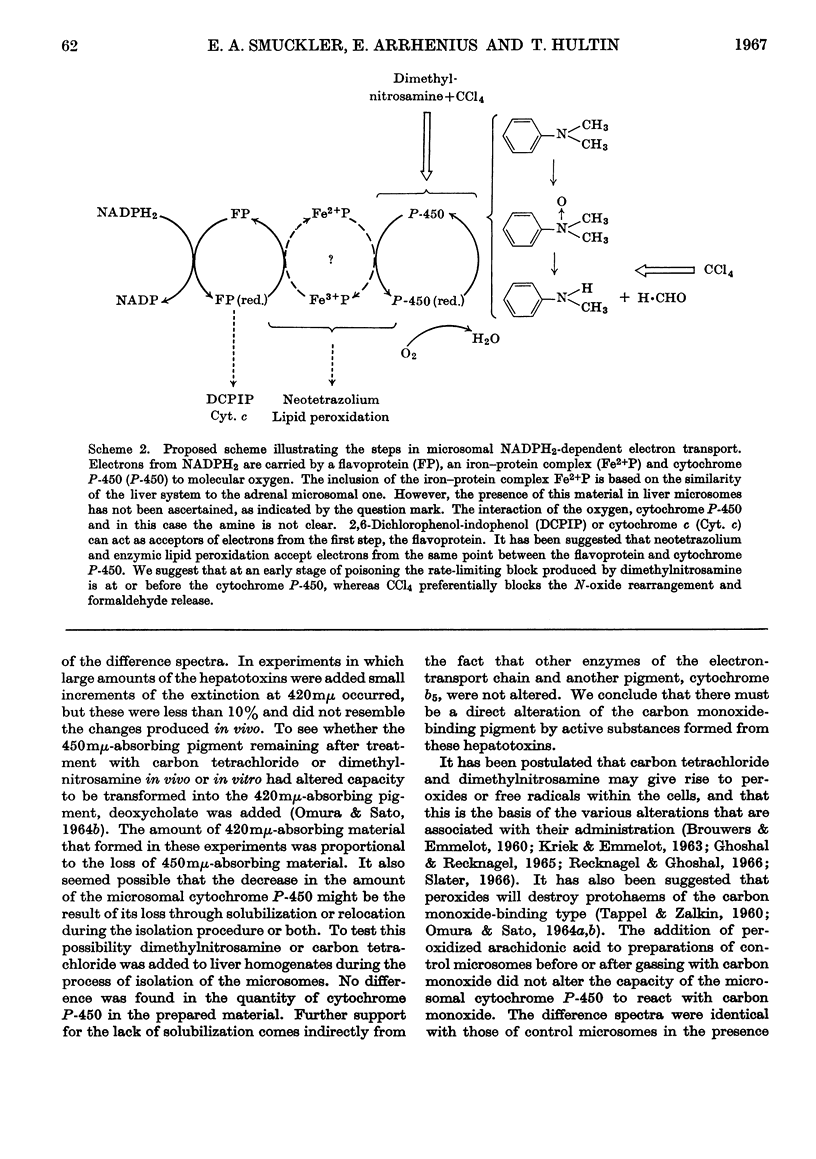
The effect of administration of carbon tetrachloride and dimethylnitrosamine in vivo on hepatic microsomal function related to drug metabolism was measured. It was found that the capacity of isolated microsomes to demethylate dimethylaniline was diminished during the first hour after carbon tetrachloride poisoning and during the second hour after dimethylnitrosamine poisoning. Thereafter the microsomes from carbon tetrachloride-poisoned livers showed a continuous decline in activity so that at 24hr. there was little residual capacity to undertake demethylation. Microsomes from dimethylnitrosamine-poisoned animals were not different from controls at 24hr. During the first 3hr. there was a transient rise in the accumulation of the N-oxide intermediate in carbon tetrachloride-poisoned livers, with a subsequent fall to below control values. In dimethylnitrosamine poisoning there was a parallel decrease in N-oxide accumulation with decreased demethylation. In the latter part of the first 24hr. the ratio of N-oxide accumulation to demethylation was increased in both instances. At 2hr. after poisoning with either compound there was no evidence of altered NADPH2-dependent neotetrazolium reduction or lipid peroxidation. NADPH2-dependent azo-dye cleavage was decreased. There was no difference in microsomal cytochrome b5 content, but there was a decrease in the amount of cytochrome P-450. This latter change was correlated with the decreased capacity for NADPH2-dependent oxidative demethylation. It is suggested that dimethylnitrosamine is associated with a defect in microsomal NADPH2-dependent electron transport at the level of cytochrome P-450. In addition to affecting cytochrome P-450, carbon tetrachloride is associated with a second severe block involving the release of formaldehyde from the N-oxide intermediate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BASSI M. Electron microscopy of rat liver after carbon tetrachloride poisoning. Exp Cell Res. 1960 Aug;20:313–323. doi: 10.1016/0014-4827(60)90160-9. [DOI] [PubMed] [Google Scholar]
- BROUWERS J. A., EMMELOT P. Microsomal N-demethylation and the effect of the hepatic carcinogen dimethylnitrosamine on amino acid incorporation into the proteins of rat livers and hepatomas. Exp Cell Res. 1960 Apr;19:467–474. doi: 10.1016/0014-4827(60)90056-2. [DOI] [PubMed] [Google Scholar]
- Cleveland P. D., Smuckler DASMUCKLER E. A. Effect of CCl4, dimethylnitrosamine, and thioacetamide on hepatic DPNH and TPNH cytochrome C reductase. Proc Soc Exp Biol Med. 1965 Dec;120(3):808–810. doi: 10.3181/00379727-120-30660. [DOI] [PubMed] [Google Scholar]
- Comporti M., Saccocci C., Dianzani M. U. Effect of CCl-4 in vitro and in vivo on lipid peroxidation of rat liver homogenates and subcellular fractions. Enzymologia. 1965 Nov 6;29(3):185–204. [PubMed] [Google Scholar]
- EL-KHATIB S., CHENAU U. A., CARPENTER M. P., TRUCCO R. E., CAPUTTO R. POSSIBLE PRESENCE OF LIPID PEROXIDES IN TISSUE AND TOCOPHEROL DEFICIENT ANIMALS. Nature. 1964 Jan 11;201:188–189. doi: 10.1038/201188a0. [DOI] [PubMed] [Google Scholar]
- EMMELOT P., BENEDETTI E. L. Changes in the fine structure of rat liver cells brought about by dimethylnitrosamine. J Biophys Biochem Cytol. 1960 Apr;7:393–396. doi: 10.1083/jcb.7.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernster L., Orrenius S. Substrate-induced synthesis of the hydroxylating enzyme system of liver microsomes. Fed Proc. 1965 Sep-Oct;24(5):1190–1199. [PubMed] [Google Scholar]
- GARFINKEL D. Studies on pig liver microsomes. I. Enzymic and pigment composition of different microsomal fractions. Arch Biochem Biophys. 1958 Oct;77(2):493–509. doi: 10.1016/0003-9861(58)90095-x. [DOI] [PubMed] [Google Scholar]
- GILLETTE J. R., BRODIE B. B., LA DU B. N. The oxidation of drugs by liver microsomes: on the role of TPNH and oxygen. J Pharmacol Exp Ther. 1957 Apr;119(4):532–540. [PubMed] [Google Scholar]
- HOCHSTEIN P., ERNSTER L. ADP-ACTIVATED LIPID PEROXIDATION COUPLED TO THE TPNH OXIDASE SYSTEM OF MICROSOMES. Biochem Biophys Res Commun. 1963 Aug 14;12:388–394. doi: 10.1016/0006-291x(63)90111-6. [DOI] [PubMed] [Google Scholar]
- HULTIN T., ARRHENIUS E. EFFECTS OF CARCINOGENIC AMINES ON AMINO ACID INCORPORATION BY LIVER SYSTEMS. 3. INHIBITION BY AMINOFLUORENE TREATMENT AND ITS DEPENDENCE ON VITAMIN E. Cancer Res. 1965 Feb;25:124–131. [PubMed] [Google Scholar]
- HULTIN T., ARRHENIUS E., LOW H., MAGEE P. N. Toxic liver injury. Inhibition by dimethylnitrosamine of incorporation of labelled amino acids into proteins of rat-liver preparations in vitro. Biochem J. 1960 Jul;76:109–116. doi: 10.1042/bj0760109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER F. E., Jr, GEBICKI J. M., HOFFSTEN P. E., WEINSTEIN J., SCOTT A. Swelling and lysis of rat liver mitochondria induced by ferrous ions. J Biol Chem. 1963 Feb;238:828–835. [PubMed] [Google Scholar]
- KLINGENBERG M. Pigments of rat liver microsomes. Arch Biochem Biophys. 1958 Jun;75(2):376–386. doi: 10.1016/0003-9861(58)90436-3. [DOI] [PubMed] [Google Scholar]
- KRIEK E., EMMELOT P. METHYLATION AND BREAKDOWN OF MICROSOMAL AND SOLUBLE RIBONUCLEIC ACID FROM RAT LIVER BY DIAZOMETHANE. Biochemistry. 1963 Jul-Aug;2:733–740. doi: 10.1021/bi00904a019. [DOI] [PubMed] [Google Scholar]
- LA DU B. N., GAUDETTE L., TROUSOF N., BRODIE B. B. Enzymatic dealkylation of aminopyrine (pyramidon) and other alkylamines. J Biol Chem. 1955 Jun;214(2):741–745. [PubMed] [Google Scholar]
- LESTER R. L., SMITH A. L. Studies on the electron transport system. 28. The mode of reduction of tetrazolium salts by beef heart mitochondria; role of coenzyme Q and other lipids. Biochim Biophys Acta. 1961 Mar 4;47:475–496. doi: 10.1016/0006-3002(61)90543-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASON H. S. Mechanisms of oxygen metabolism. Science. 1957 Jun 14;125(3259):1185–1188. doi: 10.1126/science.125.3259.1185. [DOI] [PubMed] [Google Scholar]
- MUELLER G. C., MILLER J. A. The metabolism of methylated aminoazo dyes. II. Oxidative demethylation by rat liver homogenates. J Biol Chem. 1953 Jun;202(2):579–587. [PubMed] [Google Scholar]
- Machinist J. M., Orme-Johnson W. H., Ziegler D. M. Microsomal oxidases. II. Properties of a pork liver microsomal N-oxide dealkylase. Biochemistry. 1966 Sep;5(9):2939–2943. doi: 10.1021/bi00873a025. [DOI] [PubMed] [Google Scholar]
- Mason H. S., North J. C., Vanneste M. Microsomal mixed-function oxidations: the metabolism of xenobiotics. Fed Proc. 1965 Sep-Oct;24(5):1172–1180. [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUBERT D., MAIBAUER D. Vergleichende Untersuchungen der oxydativen Leistungen von Mitochondrien und Mikrosomen bei experimenteller Leberschädigung. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1959;235(4):291–300. [PubMed] [Google Scholar]
- OBERLING C., ROUILLER C. Les effets de l'intoxication aiguë au tétrachlorure de carbone sur le foie du rat; étude au microscope électronique. Ann Anat Pathol (Paris) 1956 Oct-Dec;1(4):401–427. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Omura T., Sato R., Cooper D. Y., Rosenthal O., Estabrook R. W. Function of cytochrome P-450 of microsomes. Fed Proc. 1965 Sep-Oct;24(5):1181–1189. [PubMed] [Google Scholar]
- Orrenius S., Dallner G., Ernster L. Inhibition of the TPNH-linked lipid peroxidation of liver microsomes by drugs undergoing oxidative demethylation. Biochem Biophys Res Commun. 1964;14:329–334. doi: 10.1016/s0006-291x(64)80005-x. [DOI] [PubMed] [Google Scholar]
- RECKNAGEL R. O., LITTERIA M. Biochemical changes in carbon tetrachloride fatty liver: concentration of carbon tetrachloride in liver and blood. Am J Pathol. 1960 May;36:521–531. [PMC free article] [PubMed] [Google Scholar]
- RECKNAGEL R. O., LOMBARDI B. Studies of biochemical changes in subcellular particles of rat liver and their relationship to a new hypothesis regarding the pathogenesis of carbon tetrachloride fat accumulation. J Biol Chem. 1961 Feb;236:564–569. [PubMed] [Google Scholar]
- REYNOLDS E. S. LIVER PARENCHYMAL CELL INJURY. I. INITIAL ALTERATIONS OF THE CELL FOLLOWING POISONING WITH CARBON TETRACHLORIDE. J Cell Biol. 1963 Oct;19:139–157. doi: 10.1083/jcb.19.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recknagel R. O., Ghoshal A. K. Lipoperoxidation as a vector in carbon tetrachloride hepatotoxicity. Lab Invest. 1966 Jan;15(1 Pt 1):132–148. [PubMed] [Google Scholar]
- SMUCKLER E. A., BENDITT E. P. STUDIES ON CARBON TETRACHLORIDE INTOXICATION. 3. A SUBCELLULAR DEFECT IN PROTEIN SYNTHESIS. Biochemistry. 1965 Apr;4:671–679. doi: 10.1021/bi00880a009. [DOI] [PubMed] [Google Scholar]
- SMUCKLER E. A., ISERI O. A., BENDITT E. P. An intracellular defect in protein synthesis induced by carbon tetrachloride. J Exp Med. 1962 Jul 1;116:55–72. doi: 10.1084/jem.116.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F. Necrogenic action of carbon tetrachloride in the rat: a speculative mechanism based on activation. Nature. 1966 Jan 1;209(5018):36–40. doi: 10.1038/209036a0. [DOI] [PubMed] [Google Scholar]
- TAPPEL A., ZALKIN H. Inhibition of lipid peroxidation in microsomes by vitamin E. Nature. 1960 Jan 2;185:35–35. doi: 10.1038/185035a0. [DOI] [PubMed] [Google Scholar]
- WILBUR K. M., BERNHEIM F., SHAPIRO O. W. The thiobarbituric acid reagent as a test for the oxidation of unsaturated fatty acids by various agents. Arch Biochem. 1949 Dec;24(2):305–313. [PubMed] [Google Scholar]
- Ziegler D. M., Pettit F. H. Microsomal oxidases. I. The isolation and dialkylarylamine oxygenase activity of pork liver microsomes. Biochemistry. 1966 Sep;5(9):2932–2938. doi: 10.1021/bi00873a024. [DOI] [PubMed] [Google Scholar]


