Abstract
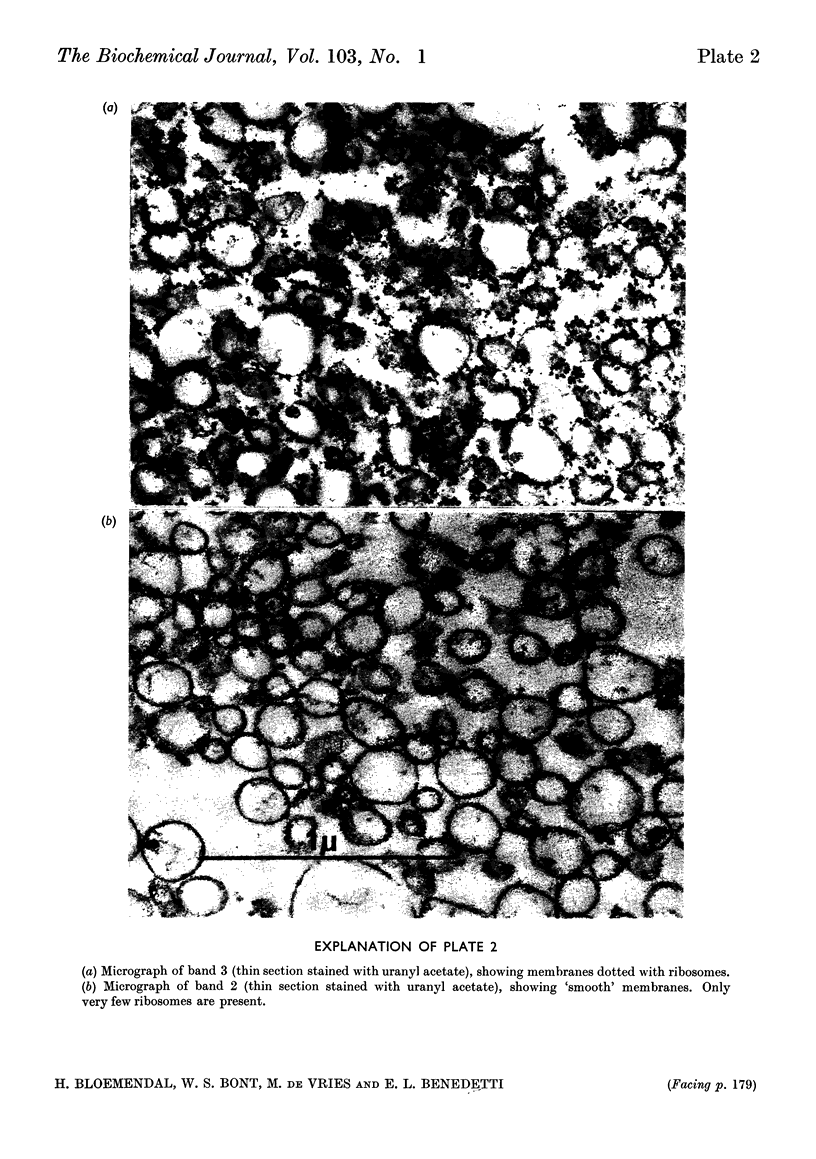
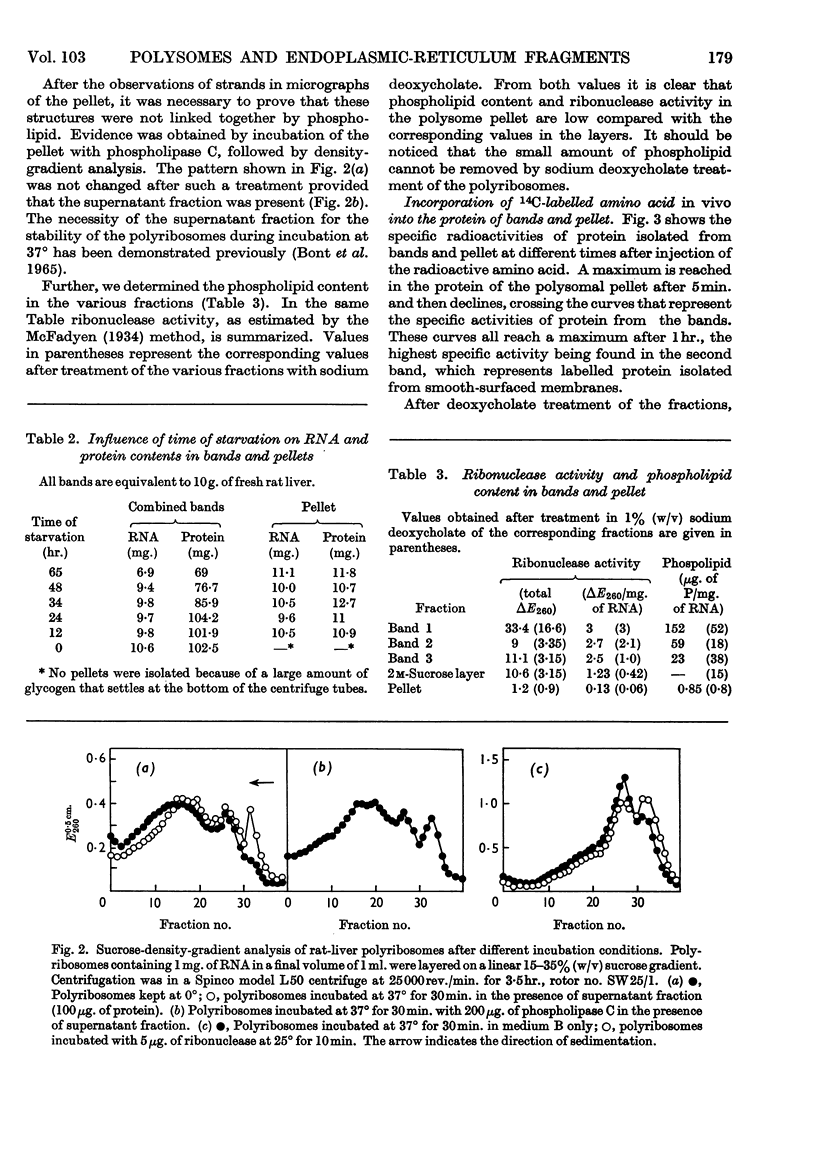
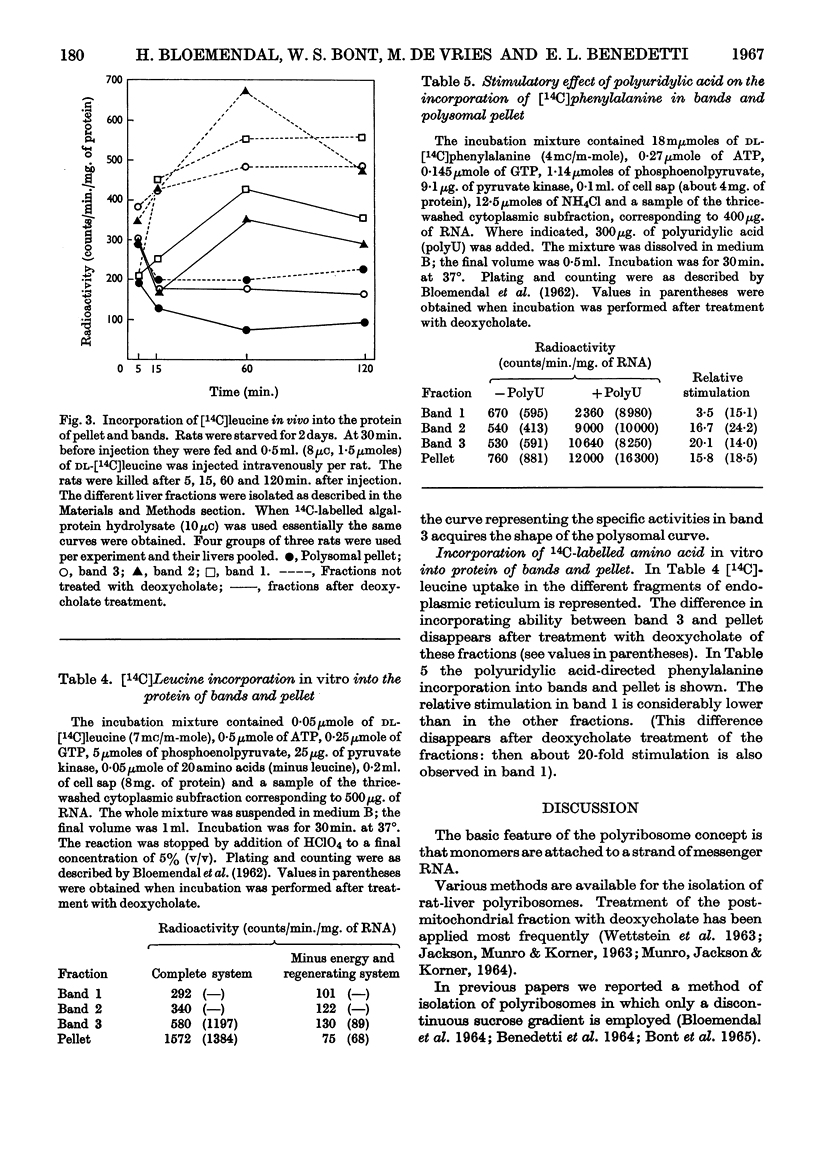
1. A centrifugation method for the fractionation of the postmitochondrial fraction from rat-liver homogenates is described. The technique, in which no detergent is used, may be used as a tool to discriminate between two classes of ribosomes. One class is firmly bound to membranes and the other consists either of free polysomes or of ribosomes attached by weaker forces to the membranes of the endoplasmic reticulum. 2. Electron-micrograph studies revealed that the polysomes were not contaminated with bound ribosomes or with membranous fragments. 3. The separated fractions were characterized by their RNA, protein, ribonuclease and phospholipid content. 4. The influence of starvation on the RNA and protein contents of the different fractions was investigated. 5. Labelling of the various centrifugal fractions in vivo revealed no difference in uptake of radioactive amino acid between the two classes of ribosomes. 6. Incorporation of radioactive leucine in vitro and the polyuridylic acid-directed phenylalanine incorporation were similar for both classes of ribosomes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENEDETTI E. L., BLOEMENDAL H., BONT W. S. POLYRIBOSOMES ISOL'ES 'A PARTIR DU FOIE DE RAT. C R Hebd Seances Acad Sci. 1964 Aug 10;259:1353–1356. [PubMed] [Google Scholar]
- BLOEMENDAL H., BONT W. S., BENEDETTI E. L. PREPARATION OF RAT-LIVER POLYSOMES WITHOUT THE UTILIZATION OF DETERGENT. Biochim Biophys Acta. 1964 May 18;87:177–180. doi: 10.1016/0926-6550(64)90064-7. [DOI] [PubMed] [Google Scholar]
- BOSCH L., HUIZINGA F., BLOEMENDAL H. Studies on cytoplasmic ribonucleic acid from rat liver. VI. Tentative mechanism of the metabolic interaction between soluble and microsomal ribonucleic acid. Biochim Biophys Acta. 1962 Aug 20;61:220–228. [PubMed] [Google Scholar]
- Benedetti E. L., Bont W. S., Bloemendal H. Electron microscopic observation on polyribosomes and endoplasmic reticulum fragments isolated from rat liver. Lab Invest. 1966 Jan;15(1 Pt 1):196–208. [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Relation of ribonuclease and ribonuclease inhibitor to the isolation of polysomes from rat liver. Proc Natl Acad Sci U S A. 1966 May;55(5):1283–1288. doi: 10.1073/pnas.55.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H., Bont W. S., Feltkamp C. A. Studies on hypophyseal isografts in mice. II. Properties of polyribosomes. Cancer Res. 1966 Jul;26(7):1497–1501. [PubMed] [Google Scholar]
- Campbell P. N., Cooper C., Hicks M. Studies on the role of the morphological constituents of the microsome fraction from rat liver in protein synthesis. Biochem J. 1964 Aug;92(2):225–234. doi: 10.1042/bj0920225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. N., Serck-Hanssen G., Lowe E. Studies on the protein-synthesizing activity of the ribosomes of rat liver. The activity of free polysomes. Biochem J. 1965 Nov;97(2):422–431. doi: 10.1042/bj0970422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- HENSHAW E. C., BOJARSKI T. B., HIATT H. H. PROTEIN SYNTHESIS BY FREE AND BOUND RAT LIVER RIBOSOMES IN VIVO AND IN VITRO. J Mol Biol. 1963 Aug;7:122–129. doi: 10.1016/s0022-2836(63)80041-8. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W., KELLER E. B., GROSS J., ZAMECNIK P. C. Studies on cytoplasmic ribonucleoprotein particles from the liver of the rat. J Biol Chem. 1955 Nov;217(1):111–123. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawford G. R., Langford P., Schachter H. The inhibition of rat liver polyribosome breakdown in the presence of liver supernatant. J Biol Chem. 1966 Apr 25;241(8):1835–1839. [PubMed] [Google Scholar]
- Manganiello V. C., Phillips A. H. The relationship between ribosomes and the endoplasmic reticulum during protein synthesis. J Biol Chem. 1965 Oct;240(10):3951–3959. [PubMed] [Google Scholar]
- Munro A. J., Jackson R. J., Korner A. Studies on the nature of polysomes. Biochem J. 1964 Aug;92(2):289–299. doi: 10.1042/bj0920289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH J. S. Ribonuclease. VII. Partial purification and characterization of a ribonuclease inhibitor in rat liver supernatant fraction. J Biol Chem. 1958 Apr;231(2):1085–1095. [PubMed] [Google Scholar]
- SIEKEVITZ P., PALADE G. E. A cyto-chemical study on the pancreas of the guinea pig. III. In vivo incorporation of leucine-1-C14 into the proteins of cell fractions. J Biophys Biochem Cytol. 1958 Sep 25;4(5):557–566. doi: 10.1083/jcb.4.5.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada K., Lieberman I. Protein synthesis by liver polyribosomes after partial hepatectomy. Biochem Biophys Res Commun. 1965 Jun 9;19(6):702–707. doi: 10.1016/0006-291x(65)90314-1. [DOI] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]





