Abstract
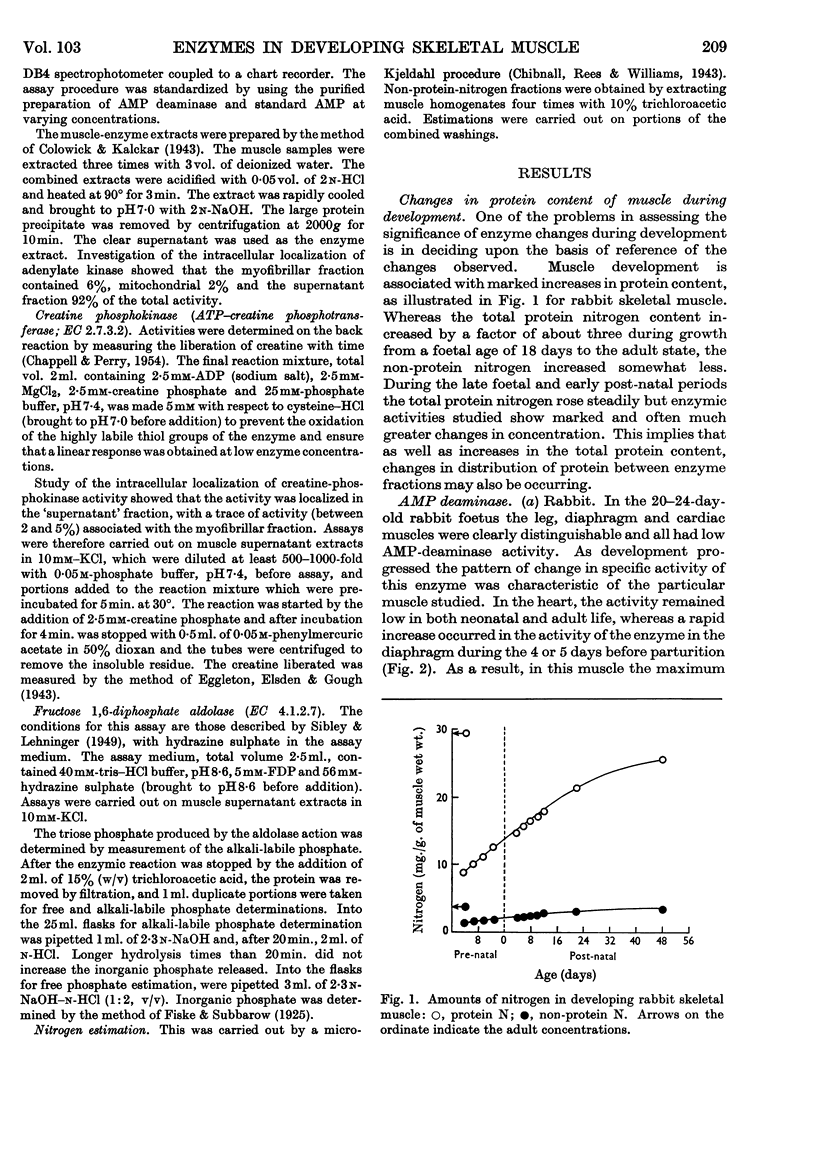
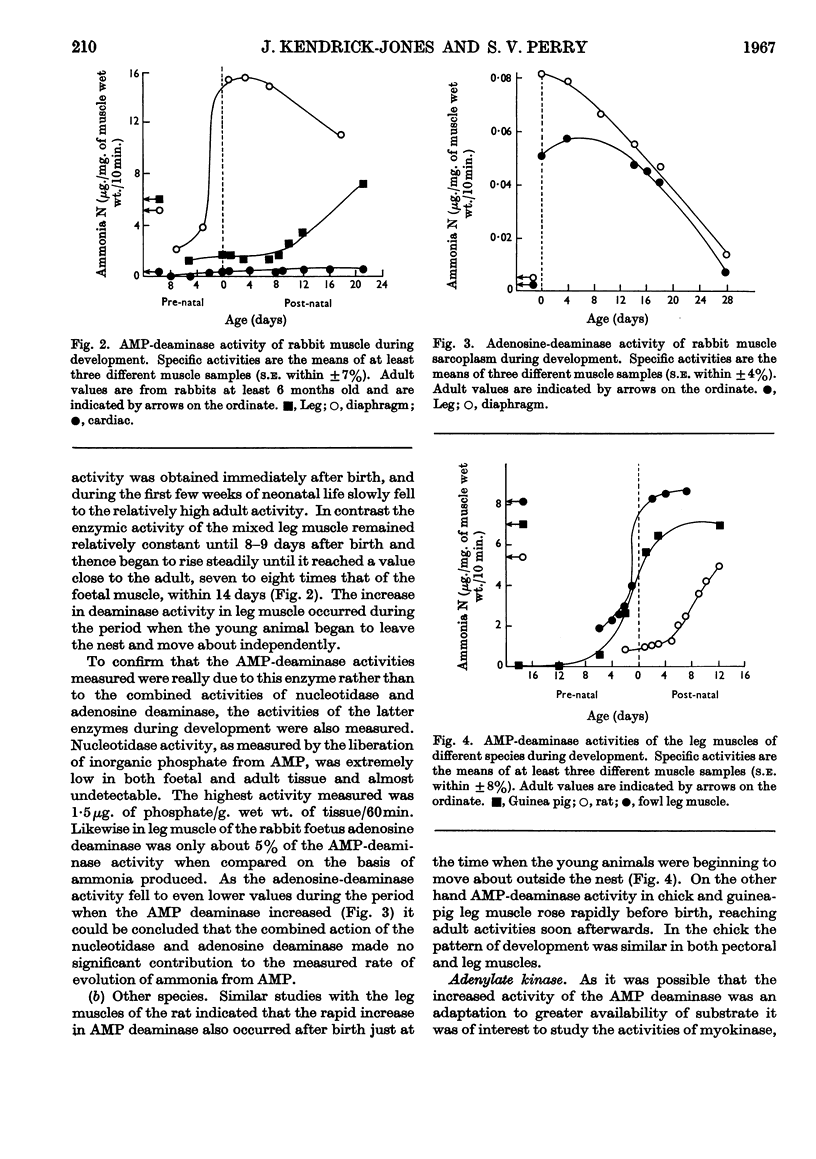
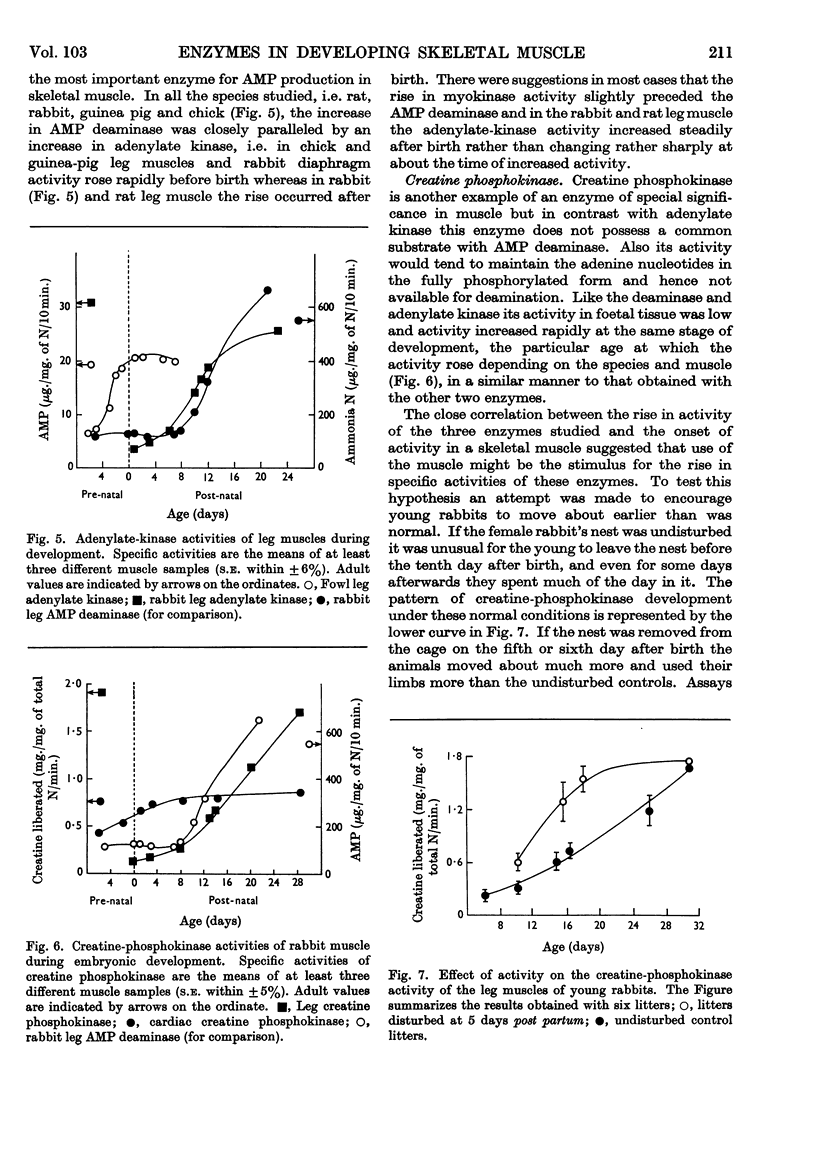
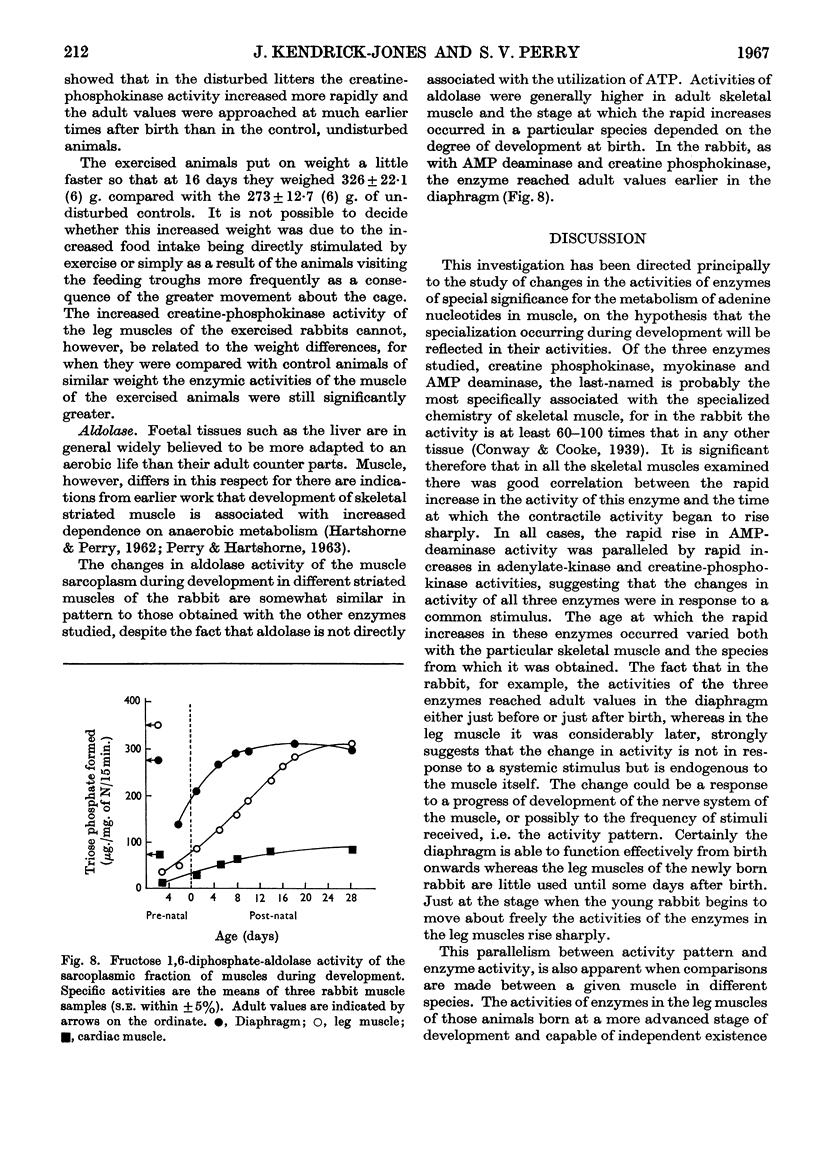
1. During late foetal and early post-natal development of rabbit skeletal muscle the total protein increased more rapidly than the non-protein nitrogen content per g. wet wt. 2. AMP-deaminase activity of rabbit leg muscles increased rapidly over the period 5–15 days after birth. In diaphragm muscle from the same animal the rapid increase to the adult enzymic activity took place at about the time of birth. 3. The rapid increase in AMP-deaminase activity of leg muscle occurred earlier in animals born relatively mature, such as the chick and guinea pig, than in animals less well developed at birth, such as the rabbit and rat. 4. The pattern of enzymic activity shown by AMP deaminase during development in diaphragm, leg and cardiac muscles in a given species was closely paralleled by those of adenylate kinase and creatine phosphokinase. 5. When young rabbits were encouraged to become active at an earlier stage than is normal, the rise in creatine-phosphokinase activity occurred at an earlier age than in the control animals. 6. The results suggest that the activity pattern of the muscle is an important factor in determining the time at which the activities of the enzymes of special significance for muscle rise sharply to the adult values. 7. Development in rabbit leg muscle also involved an increase in aldolase activity. The pattern of change was similar to that obtained with other enzymes studied.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAPPELL J. B., PERRY S. V. Creatine phosphokinase: assay and application for the micro-determination of the adenine nucleotides. Biochem J. 1954 Jul;57(3):421–427. doi: 10.1042/bj0570421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn R. D., Zwilling E., Kaplan N. O., Levine L. Nature and Development of Lactic Dehydrogenases: The two major types of this enzyme form molecular hybrids which change in makeup during development. Science. 1962 Jun 15;136(3520):962–969. doi: 10.1126/science.136.3520.962. [DOI] [PubMed] [Google Scholar]
- Chibnall A. C., Rees M. W., Williams E. F. The total nitrogen content of egg albumin and other proteins. Biochem J. 1943 Sep;37(3):354–359. doi: 10.1042/bj0370354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. J., Cooke R. The deaminases of adenosine and adenylic acid in blood and tissues. Biochem J. 1939 Apr;33(4):479–492. doi: 10.1042/bj0330479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., TRUDGILL P. W. THE METABOLISM OF GALACTARATE, D-GLUCARATE AND VARIOUS PENTOSES BY SPECIES OF PSEUDOMONAS. Biochem J. 1965 Apr;95:48–58. doi: 10.1042/bj0950048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleton P., Elsden S. R., Gough N. The estimation of creatine and of diacetyl. Biochem J. 1943;37(5):526–529. doi: 10.1042/bj0370526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTSHORNE D. J., PERRY S. V. A chromatographic and electrophoretic study of sarcoplasm from adult--and foetal-rabbit muscles. Biochem J. 1962 Oct;85:171–177. doi: 10.1042/bj0850171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRMANN H., COX W. M. Content of inorganic phosphate and phosphate esters in muscle tissue of chick embryo. Am J Physiol. 1951 Jun;165(3):711–715. doi: 10.1152/ajplegacy.1951.165.3.711. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Perry S. V. Enzymatic adaptation to contractile activity in skeletal muscle. Nature. 1965 Dec 11;208(5015):1068–1070. doi: 10.1038/2081068a0. [DOI] [PubMed] [Google Scholar]
- LINDSAY D. T. Isozymic patterns and properties of lactate dehydrogenase from developing tissues of the chicken. J Exp Zool. 1963 Feb;152:75–89. doi: 10.1002/jez.1401520108. [DOI] [PubMed] [Google Scholar]
- PERRY S. V., ZYDOWO M. The nature of the extra protein fraction from myofibrils of striated muscle. Biochem J. 1959 Feb;71(2):220–228. doi: 10.1042/bj0710220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- READ W. O., JOHNSON D. C. Creatine phosphokinase activity in heart and skeletal muscle of foetal rabbits. Proc Soc Exp Biol Med. 1959 Oct-Dec;102:740–741. doi: 10.3181/00379727-102-25382. [DOI] [PubMed] [Google Scholar]
- REPORTER M. C., KONIGSBERG I. R., STREHLER B. L. Kinetics of accumulation of creatine phosphokinase activity in developing embryonic skeletal muscle in vivo and in monolayer culture. Exp Cell Res. 1963 Apr;30:410–417. doi: 10.1016/0014-4827(63)90313-6. [DOI] [PubMed] [Google Scholar]
- STAVE U. AGE-DEPENDENT CHANGES OF METABOLISM. I. STUDIES OF ENZYME PATTERNS OF RABBIT ORGANS. Biol Neonat. 1964;6:128–147. [PubMed] [Google Scholar]


