Abstract
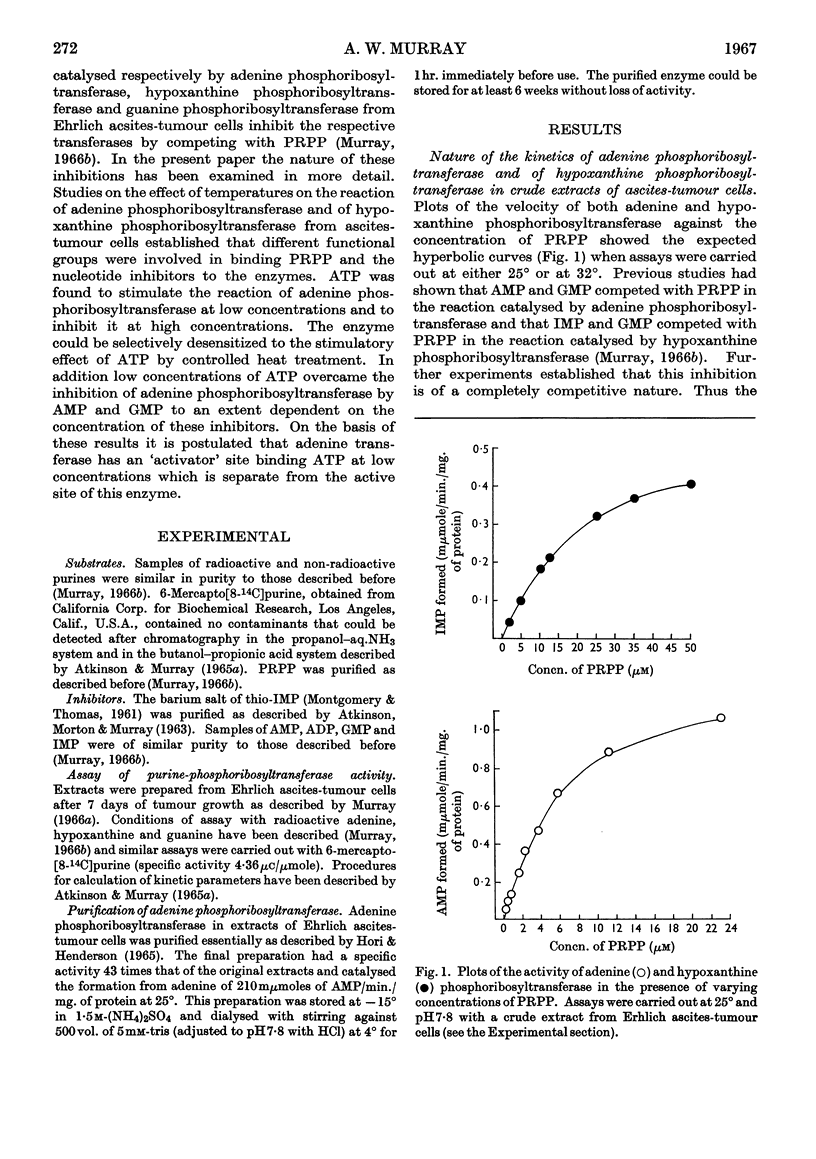
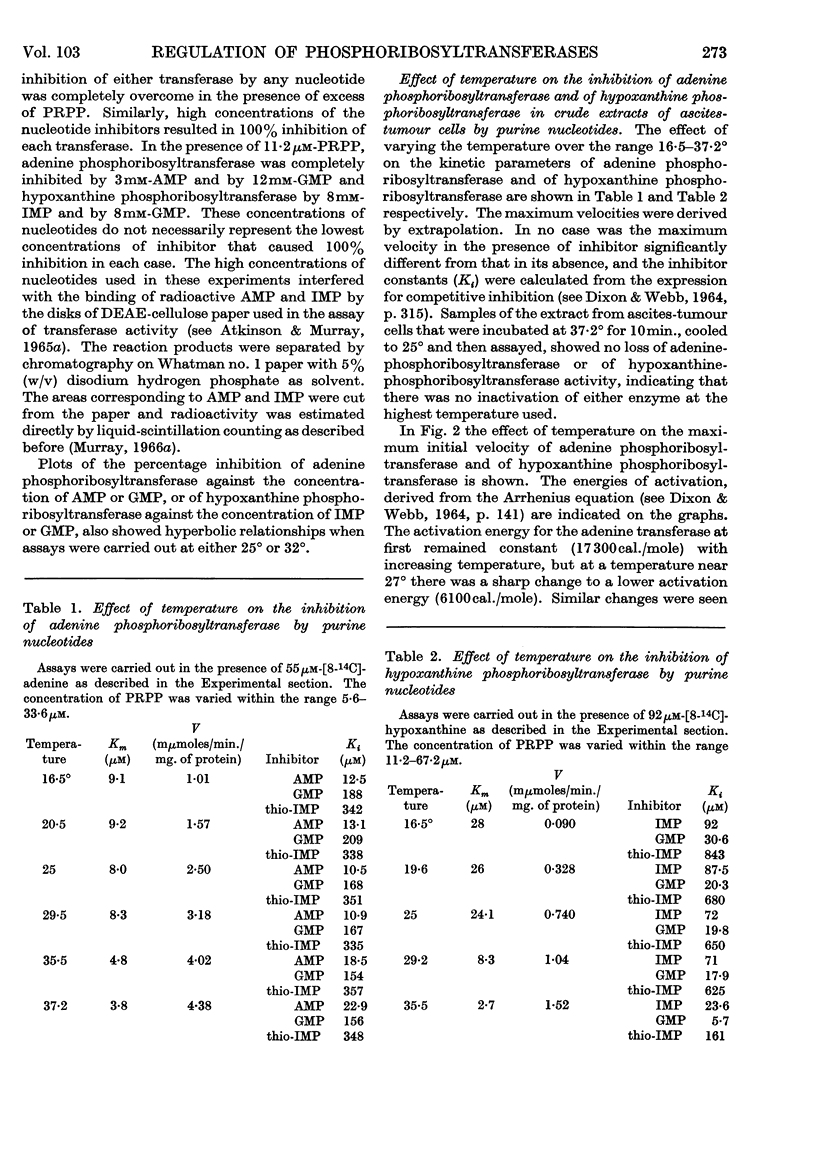
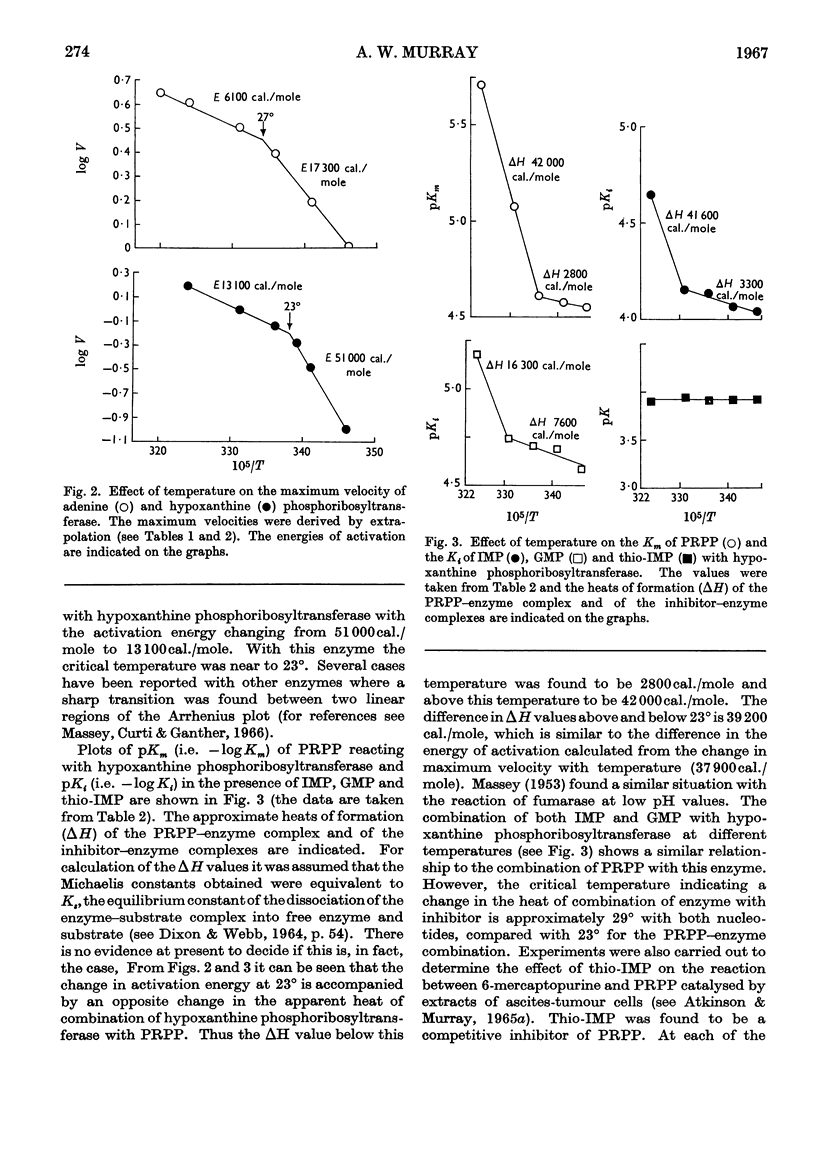
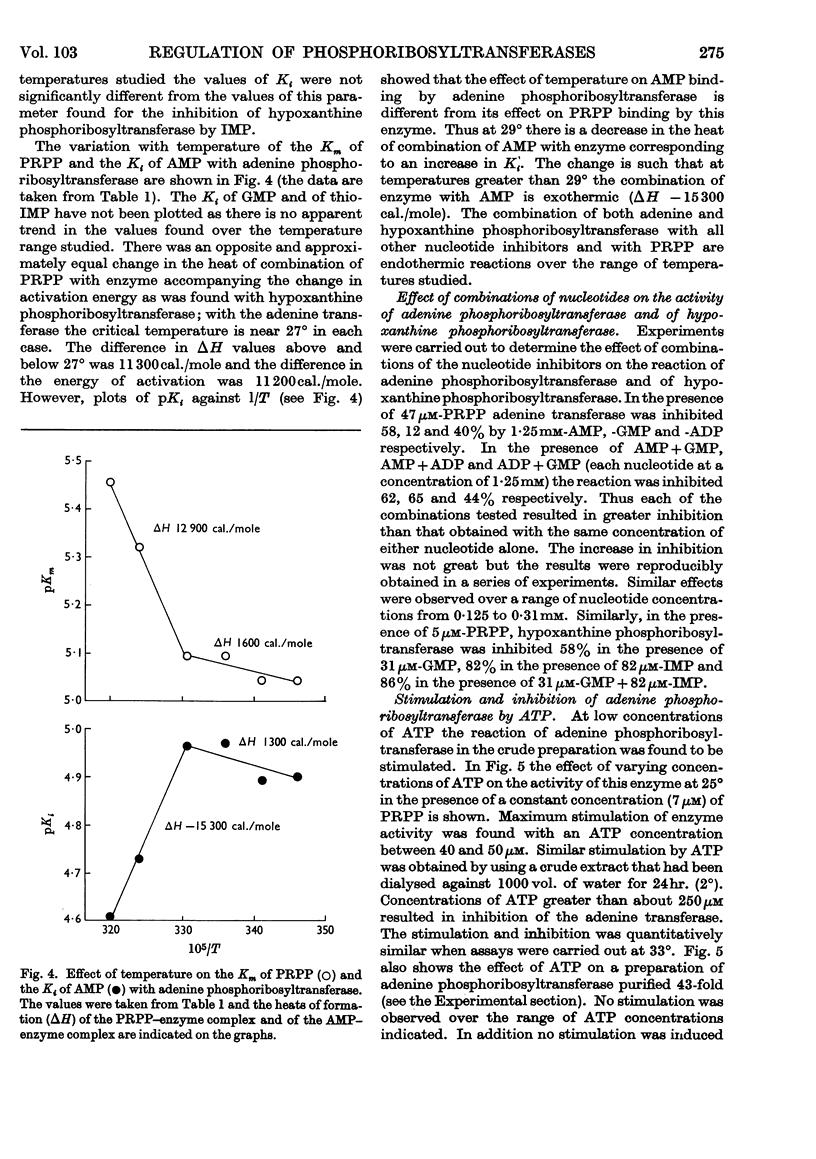
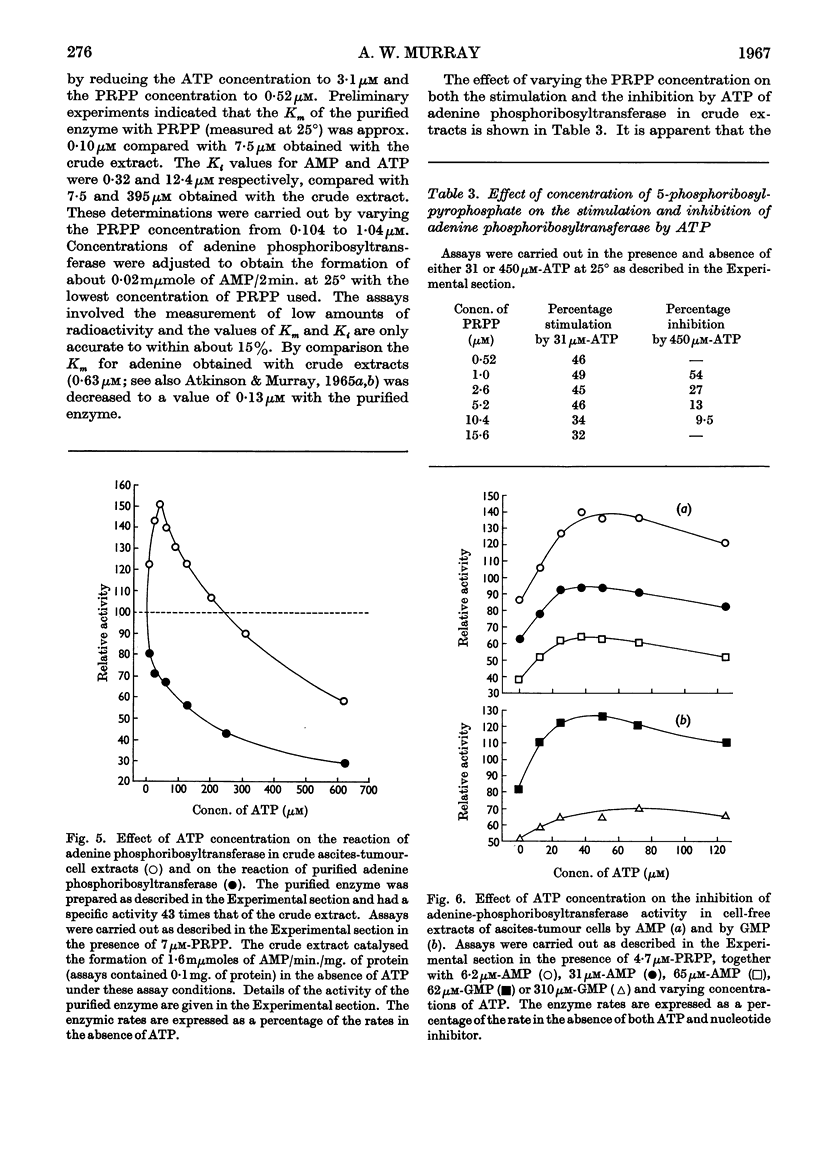
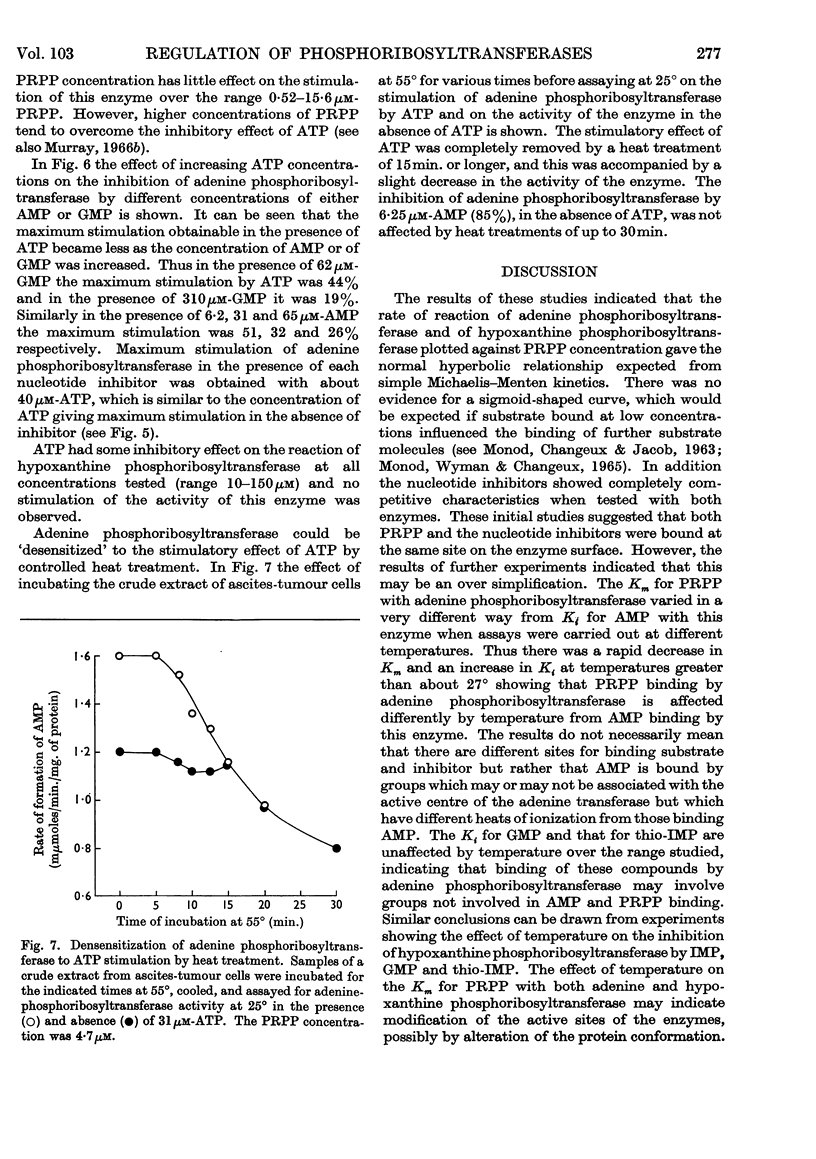
1. The progress curves of adenine phosphoribosyltransferase and of hypoxanthine phosphoribosyltransferase activity plotted against 5-phosphoribosyl pyrophosphate concentration were hyperbolic in nature. The inhibition of the former enzyme by AMP and GMP and of the latter enzyme by IMP and GMP showed completely competitive characteristics. 2. The effect of temperature on the reaction of adenine phosphoribosyltransferase and of hypoxanthine phosphoribosyltransferase was examined. The energy of activation of the former enzyme decreased at temperatures greater than 27° and that of the latter enzyme at temperatures greater than 23°. For each enzyme, the change in the heat of formation of the 5-phosphoribosyl pyrophosphate–enzyme complex at the critical temperature was approximately equal to the change in the energy of activation but was in the opposite direction. The inhibitor constants with both enzymes in the presence of nucleotides varied in different ways with temperature from the Michaelis constants for 5-phosphoribosyl pyrophosphate indicating that different functional groups were involved in binding substrates and inhibitors. 3. ATP was found to stimulate adenine-phosphoribosyltransferase activity at concentrations less than about 250μm and to inhibit the enzyme at concentrations greater than 250μm. The stimulation was unaffected by 5-phosphoribosyl pyrophosphate concentration but the inhibitory effect could be overcome by increasing concentrations of this compound. At low concentrations ATP reversed the inhibition of adenine phosphoribosyltransferase by AMP and GMP to an extent dependent on their concentration. 4. The properties of adenine phosphoribosyltransferase changed markedly on purification. Crude extracts of ascites-tumour cells had Michaelis constants for 5-phosphoribosyl pyrophosphate and adenine 75 and six times as high respectively as those obtained with purified enzyme. ATP had no stimulatory effect on activity of the purified enzyme or on that of crude extracts heated 15min. or longer at 55°. 5. It is suggested that at low concentrations ATP is bound to an `activator' site which is separate from the substrate binding site of adenine phosphorytransferase and that at high concentrations ATP competes with 5-phosphoribosyl pyrophosphate at the active site of the enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON M. R., MORTON R. K., MURRAY A. W. INHIBITION OF INOSINE 5'-PHOSPHATE DEHYDROGENASE FROM EHRLICH ASCITES-TUMOUR CELLS BY 6-THIONINOSINE 5'-PHOSPHATE. Biochem J. 1963 Oct;89:167–172. doi: 10.1042/bj0890167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATKINSON M. R., MURRAY A. W. INHIBITION BY 6-MERCAPTOPURINE OF PURINE PHOSPHORIBOSYLTRANSFERASES FROM EHRLICH ASCITES-TUMOUR CELLS THAT ARE RESISTANT TO THE DRUG. Biochem J. 1965 Jan;94:71–74. doi: 10.1042/bj0940071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATKINSON M. R., MURRAY A. W. INHIBITION OF PRUINE PHOSPHORIBOSYLTRANSFERASES OF EHRLICH ASCITES-TUMOUR CELLS BY 6-MERCAPTOPURINE. Biochem J. 1965 Jan;94:64–70. doi: 10.1042/bj0940064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman R. W. Resistance to purine antagonists in experimental leukemia systems. Cancer Res. 1965 Oct;25(9):1596–1605. [PubMed] [Google Scholar]
- CHANGEUX J. P. The feedback control mechanisms of biosynthetic L-threonine deaminase by L-isoleucine. Cold Spring Harb Symp Quant Biol. 1961;26:313–318. doi: 10.1101/sqb.1961.026.01.037. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. ASPARTATE TRANSCARBAMYLASE, AN ENZYME DESIGNED FOR FEEDBACK INHIBITION. Fed Proc. 1964 May-Jun;23:727–735. [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Hori M., Henderson J. F. Purification and properties of adenylate pyrophosphorylase from Ehrlich ascites tumor cells. J Biol Chem. 1966 Mar 25;241(6):1406–1411. [PubMed] [Google Scholar]
- MAGER J., MAGASANIK B. Guanosine 5'-phosphate reductase and its role in the interconversion of purine nucleotides. J Biol Chem. 1960 May;235:1474–1478. [PubMed] [Google Scholar]
- MASSEY V. Studies on fumarase. III. The effect of temperature. Biochem J. 1953 Jan;53(1):72–79. doi: 10.1042/bj0530072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- Massey V., Curti B., Ganther H. A temperature-dependent conformational change in D-amino acid oxidase and its effect on catalysis. J Biol Chem. 1966 May 25;241(10):2347–2357. [PubMed] [Google Scholar]
- Murray A. W. Inhibition of purine phosphoribosyltransferases from Ehrlich ascites-tumour cells by purine nucleotides. Biochem J. 1966 Sep;100(3):671–674. doi: 10.1042/bj1000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W. Purine-phosphoribosyltransferase activities in rat and mouse tissues and in Ehrlich ascites-tumour cells. Biochem J. 1966 Sep;100(3):664–670. doi: 10.1042/bj1000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W. The effect of 6-mercaptopurine on the total acid-soluble and nucleic acid adenine and guanine nucleotides of Ehrlich ascites tumour cells. Biochim Biophys Acta. 1966 Aug 17;123(2):428–429. doi: 10.1016/0005-2787(66)90297-8. [DOI] [PubMed] [Google Scholar]
- NIERLICH D. P., MAGASANIK B. REGULATION OF PURINE RIBONUCLEOTIDE SYNTHESIS BY END PRODUCT INHIBITION. THE EFFECT OF ADENINE AND GUANINE RIBONUCLEOTIDES ON THE 5'-PHOSPHORIBOSYL-PYROPHOSPHATE AMIDOTRANSFERASE OF AEROBACTER AEROGENES. J Biol Chem. 1965 Jan;240:358–365. [PubMed] [Google Scholar]
- WYNGAARDEN J. B., GREENLAND R. A. The inhibition of succinoadenylate kinosynthetase of Escherichia coli by adenosine and guanosine 5'-monophosphates. J Biol Chem. 1963 Mar;238:1054–1057. [PubMed] [Google Scholar]


