Abstract
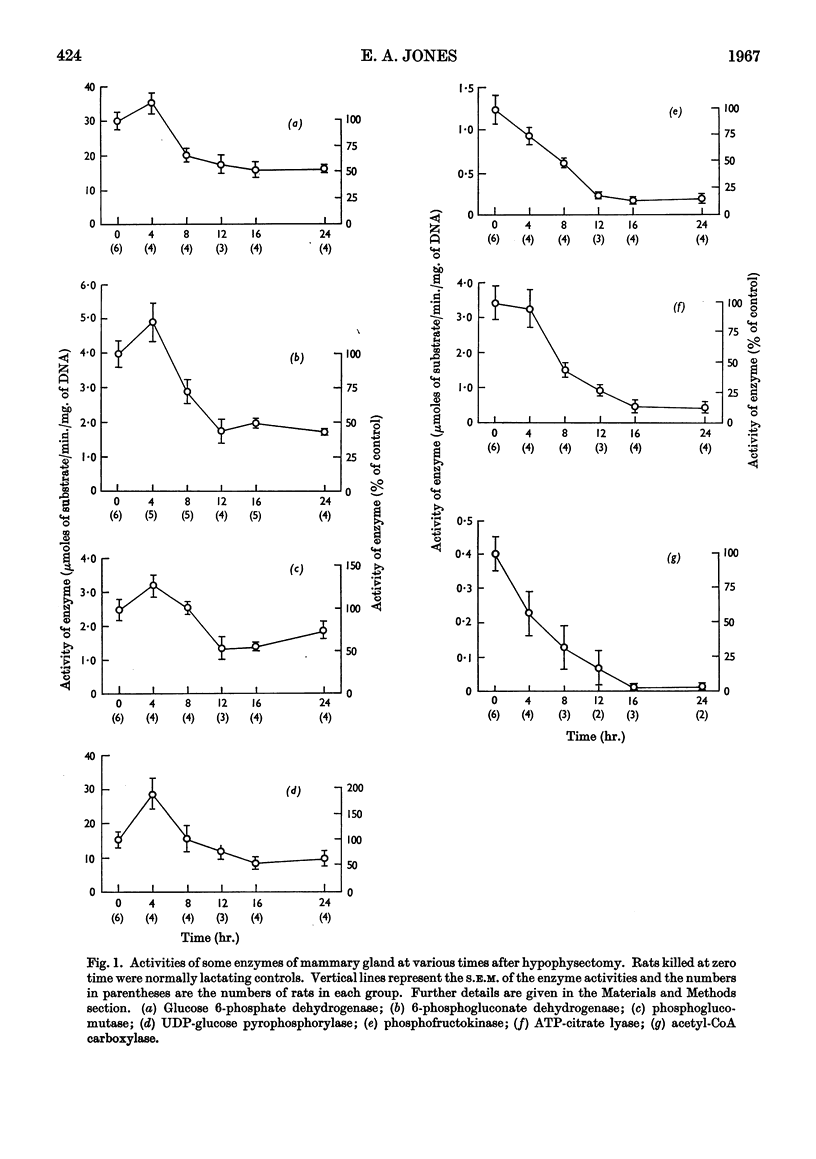
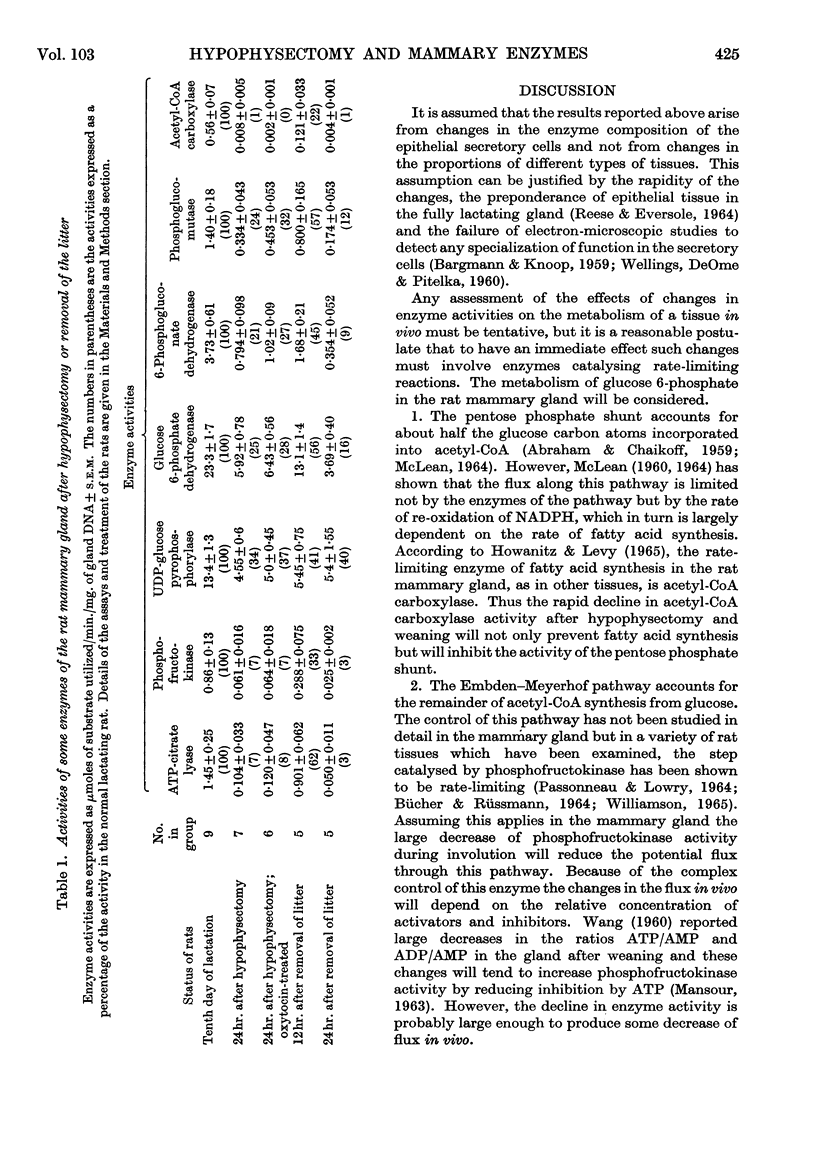
1. The enzymes glucose 6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, phosphoglucomutase, UDP-glucose pyrophosphorylase, phosphofructokinase, ATP-citrate lyase and acetyl-CoA carboxylase have been assayed in rat mammary glands in various stages of involution after hypophysectomy and weaning. 2. After hypophysectomy all seven enzymes decline in activity over a 12–16hr. period but the extent of the decline varies, with acetyl-CoA carboxylase becoming almost totally inactive, ATP-citrate lyase and phosphofructokinase showing a large decrease, and the remaining enzymes a less marked decline. 3. Within 24hr. of removing the litter a change in the pattern of enzyme activity is found very similar to that after hypophysectomy. 4. The significance of these results is discussed in relation to the endocrine control of mammary gland metabolism and the mechanisms of involution.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM S., CADY P., CHAIKOFF I. L. Glucose and acetate metabolism and lipogensis in mammary glands of hypophysectomized rats in which lactation was hormonally induced. Endocrinology. 1960 Feb;66:280–288. doi: 10.1210/endo-66-2-280. [DOI] [PubMed] [Google Scholar]
- ABRAHAM S., CHAIKOFF I. L. Glycolytic pathways and lipogenesis in mammary glands of lactating and nonlactating normal rats. J Biol Chem. 1959 Sep;234:2246–2253. [PubMed] [Google Scholar]
- BARGMANN W., KNOOP A. Uber die Morphologie der Milchsekretion; lichtund elektronenmikroskopische Studien an der Milchdrüse Ratte. Z Zellforsch Mikrosk Anat. 1959;49(3):344–388. [PubMed] [Google Scholar]
- BRADLEY T. R., COWIE A. T. The effects of hypophysectomy on the in vitro metabolism of mammary gland slices from lactating rats. J Endocrinol. 1956 Aug;14(1):8–15. doi: 10.1677/joe.0.0140008. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. L., Milligan L. P. Enzymatic changes associated with the initiation and maintenance of lactation in the rat. J Biol Chem. 1966 May 10;241(9):2058–2066. [PubMed] [Google Scholar]
- ESTABROOK R. W., MAITRA P. K. A fluorimetric method for the quantitative microanalysis of adenine and pyridine nucleotides. Anal Biochem. 1962 May;3:369–382. doi: 10.1016/0003-2697(62)90065-9. [DOI] [PubMed] [Google Scholar]
- FOLLEY S. J., FRENCH T. H. The intermediary metabolism of the mammary gland; respiration and acid production of mammary tissue during pregnancy, lactation and involution in the rat. Biochem J. 1949;45(3):270–275. doi: 10.1042/bj0450270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folley S. J., Greenbaum A. L. Changes in the arginase and alkaline phosphatase contents of the mammary gland and liver of the rat during pregnancy, lactation and mammary involution. Biochem J. 1947;41(2):261–269. doi: 10.1042/bj0410261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folley S. J., Watson S. C. A high-speed tissue homogenizer. Biochem J. 1948;42(2):204–206. doi: 10.1042/bj0420204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBAUM A. L., GREENWOOD F. C. Some enzymic changes in the mammary gland of rats during pregnancy, lactation and mammary involution. Biochem J. 1954 Apr;56(4):625–631. doi: 10.1042/bj0560625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBAUM A. L., SLATER T. F. Studies on the particulate components of rat mammary gland. II. Changes in the levels of the nucleic acids of the mammary glands of rats during pregnancy, lactation and mammary involution. Biochem J. 1957 May;66(1):155–161. doi: 10.1042/bj0660155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBAUM A. L., SLATER T. F., WANG D. Y. Lysosomal-like particles in the rat mammary gland. Nature. 1960 Oct 22;188:318–320. doi: 10.1038/188318a0. [DOI] [PubMed] [Google Scholar]
- Greenbaum A. L., Darby F. J. The effect of adrenalectomy on the metabolism of the mammary glands of lactating rats. Biochem J. 1964 May;91(2):307–317. doi: 10.1042/bj0910307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutfreund H., Jones E. A. The kinetic behaviour of enzymes in organized systems. Mitochondrial succinate oxidase and fumarase. Biochem J. 1964 Jan;90(1):208–213. doi: 10.1042/bj0900208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howanitz P. J., Levy H. R. Acetyl-CoA carboxylase and citrate cleavage enzyme in the rat mammary gland. Biochim Biophys Acta. 1965 Oct 4;106(2):430–433. doi: 10.1016/0005-2760(65)90056-1. [DOI] [PubMed] [Google Scholar]
- LYONS W. R., LI C. H., JOHNSON R. E. The hormonal control of mammary growth and lactation. Recent Prog Horm Res. 1958;14:219–254. [PubMed] [Google Scholar]
- MALPRESS F. H. Quantiative studies on mammary-gland enzymes involved in lactose synthesis. Biochem J. 1961 Mar;78:527–530. doi: 10.1042/bj0780527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNFORD R. E. CHANGES IN THE MAMMARY GLANDS OF RATS AND MICE DURING PREGNANCY, LACTATION AND INVOLUTION. 2. LEVELS OF DEOXYRIBONUCLEIC ACID, AND ALKALINE AND ACID PHOSPHATASES. J Endocrinol. 1963 Dec;28:17–34. doi: 10.1677/joe.0.0280017. [DOI] [PubMed] [Google Scholar]
- McLEAN P. Carbohydrate metabolism of mammary tissue. I. Pathways of glucose catabolism in the mammary gland. Biochim Biophys Acta. 1958 Nov;30(2):303–315. doi: 10.1016/0006-3002(58)90055-6. [DOI] [PubMed] [Google Scholar]
- McLean P. Interrelationship of carbohydrate and fat metabolism in the involuting mammary gland. Biochem J. 1964 Feb;90(2):271–278. doi: 10.1042/bj0900271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meites J., Nicoll C. S. Adenohypophysis:prolactin. Annu Rev Physiol. 1966;28:57–88. doi: 10.1146/annurev.ph.28.030166.000421. [DOI] [PubMed] [Google Scholar]
- Passonneau J. V., Lowry O. H. The role of phosphofructokinase in metabolic regulation. Adv Enzyme Regul. 1964;2:265–274. doi: 10.1016/s0065-2571(64)80018-2. [DOI] [PubMed] [Google Scholar]
- REES E. D., EVERSOLE A. RAT MAMMARY GLAND METABOLISM RELATIVE TO EPITHELIAL AND CONNECTIVE TISSUE CONTENT. Am J Physiol. 1964 Sep;207:595–600. doi: 10.1152/ajplegacy.1964.207.3.595. [DOI] [PubMed] [Google Scholar]
- RIVERA E. M. DIFFERENTIAL RESPONSIVENESS TO HORMONES OF C3H AND A MOUSE MAMMARY TISSUES IN ORGAN CULTURE. Endocrinology. 1964 Jun;74:853–864. doi: 10.1210/endo-74-6-853. [DOI] [PubMed] [Google Scholar]
- SHATTON J. B., GRUENSTEIN M., SHAY H., WEINHOUSE S. ENZYMES OF GALACTOSE SYNTHESIS IN MAMMARY GLAND AND MAMMARY TUMORS OF THE RAT. J Biol Chem. 1965 Jan;240:22–28. [PubMed] [Google Scholar]
- SLATER T. F. Studies on mammary involution. I. Chemical changes. Arch Int Physiol Biochim. 1962 Mar;70:167–178. doi: 10.3109/13813456209092850. [DOI] [PubMed] [Google Scholar]
- SRERE P. A. The citrate cleavage enzyme. I. Distribution and purification. J Biol Chem. 1959 Oct;234:2544–2547. [PubMed] [Google Scholar]
- Spencer A. F., Lowenstein J. M. Citrate and the conversion of carbohydrate into fat. Citrate cleavage in obesity and lactation. Biochem J. 1966 Jun;99(3):760–765. doi: 10.1042/bj0990760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer A., Corman L., Lowenstein J. M. Citrate and the conversion of carbohydrate into fat. A comparison of citrate and acetate incorporation into fatty acids. Biochem J. 1964 Nov;93(2):378–388. doi: 10.1042/bj0930378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUCKER H. A., REECE R. P. Nucleic acid estimates of mammary tissue and nuclei. Proc Soc Exp Biol Med. 1962 Dec;111:639–642. [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAITE M., WAKIL S. J. Studies on the mechanism of fatty acid synthesis. XII. Acetyl coenzyme A carboxylase. J Biol Chem. 1962 Sep;237:2750–2757. [PubMed] [Google Scholar]
- WANG D. Y. Effects of short-term weaning on the acid-soluble ribonucleotides of rat mammary gland. Nature. 1960 Dec 24;188:1109–1110. doi: 10.1038/1881109a0. [DOI] [PubMed] [Google Scholar]
- WELLINGS S. R., DEOME K. B., PITELKA D. R. Electron microscopy of milk secretion in the mammary gland of the C3H/Crgl mouse. I. Cytomorphology of the prelactating and the lactating gland. J Natl Cancer Inst. 1960 Aug;25:393–421. [PubMed] [Google Scholar]
- WILLIAMSON J. R. GLYCOLYTIC CONTROL MECHANISMS. I. INHIBITION OF GLYCOLYSIS BY ACETATE AND PYRUVATE IN THE ISOLATED, PERFUSED RAT HEART. J Biol Chem. 1965 Jun;240:2308–2321. [PubMed] [Google Scholar]
- WILLMER J. S. The influence of adrenalectomy upon the activity of the hexosemonophosphate shunt in the livers and mammary glands of lactating rats. Can J Biochem Physiol. 1960 Nov;38:1265–1273. [PubMed] [Google Scholar]


