Abstract
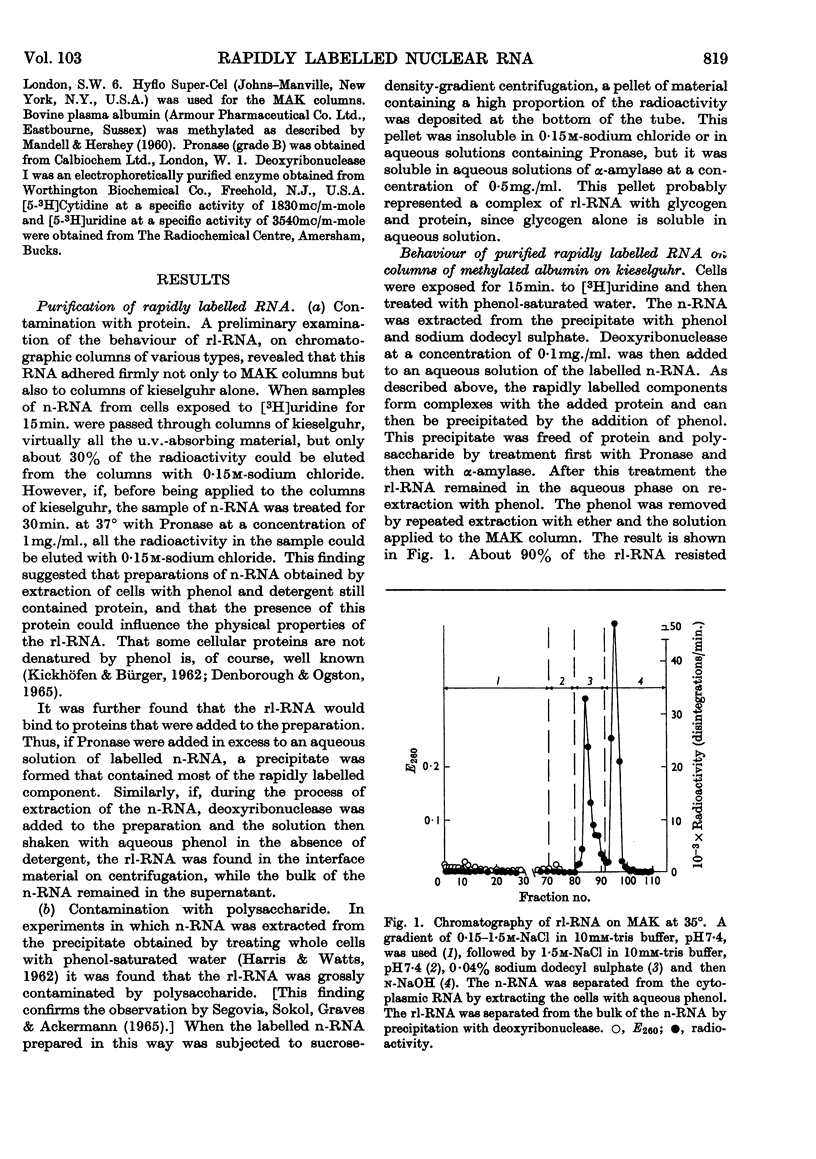
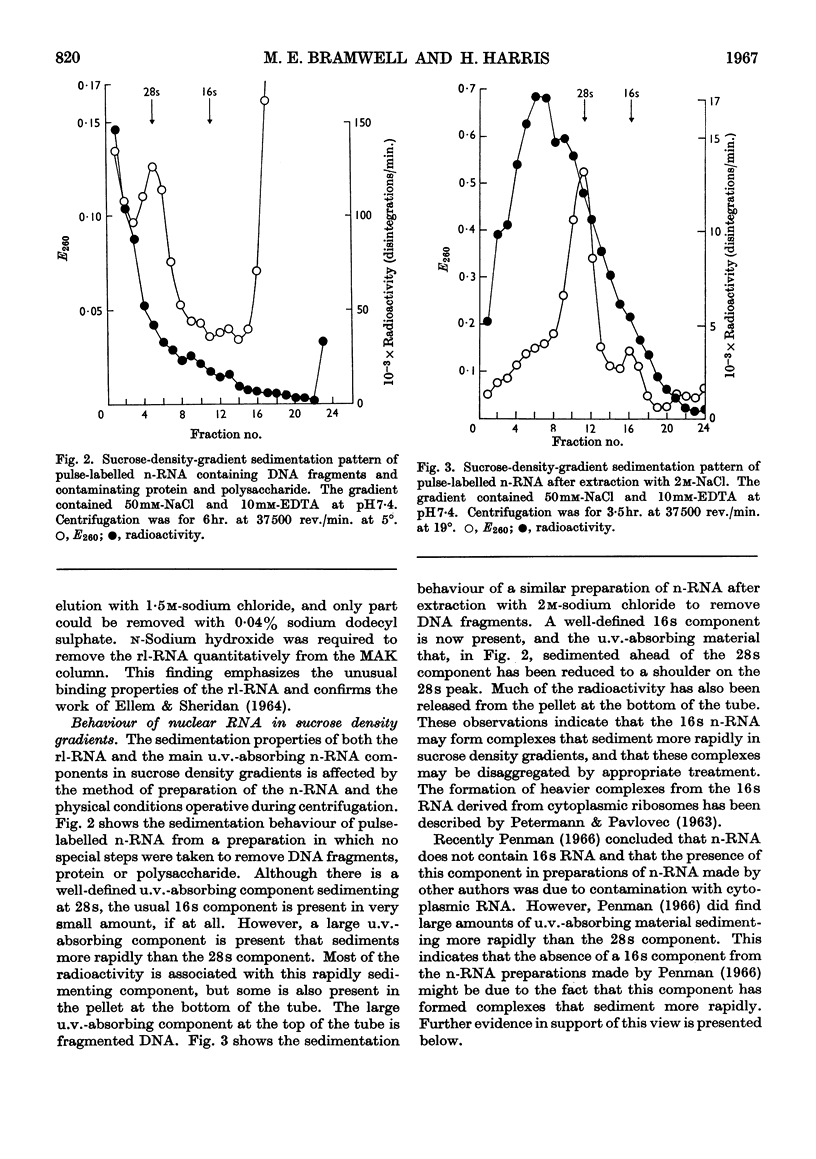
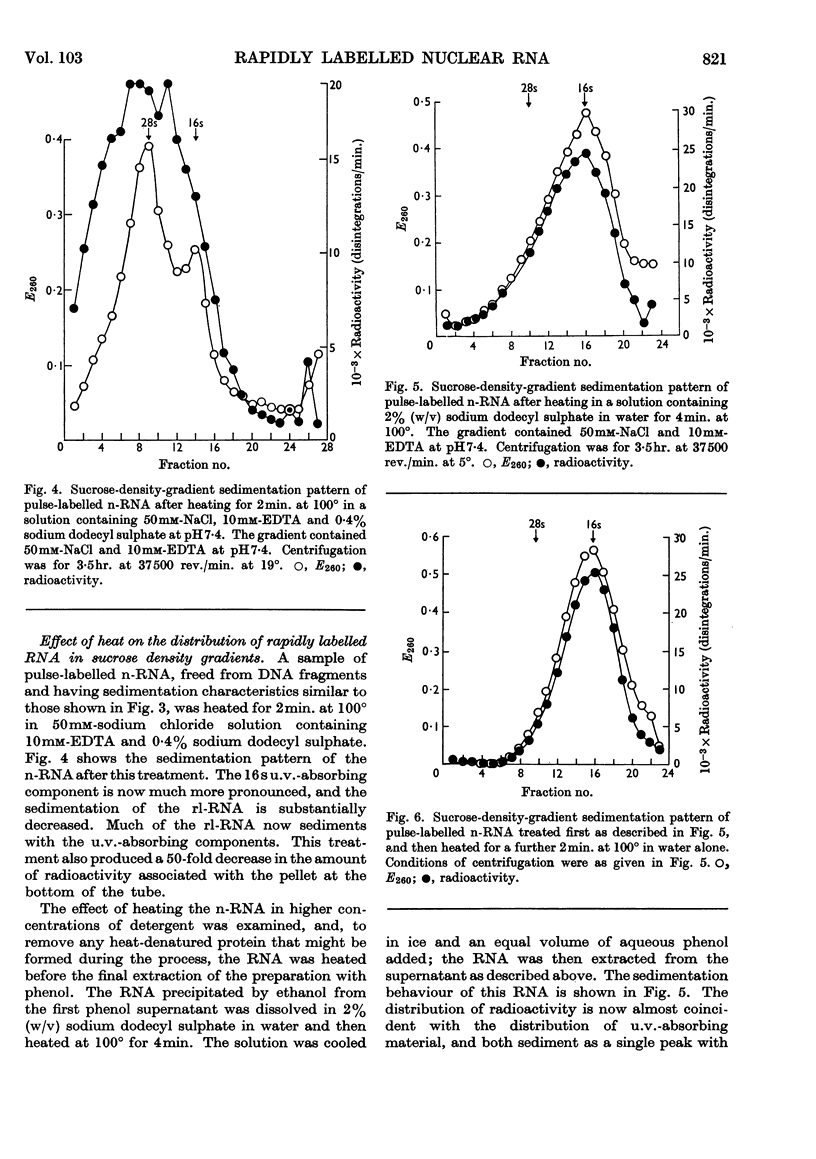
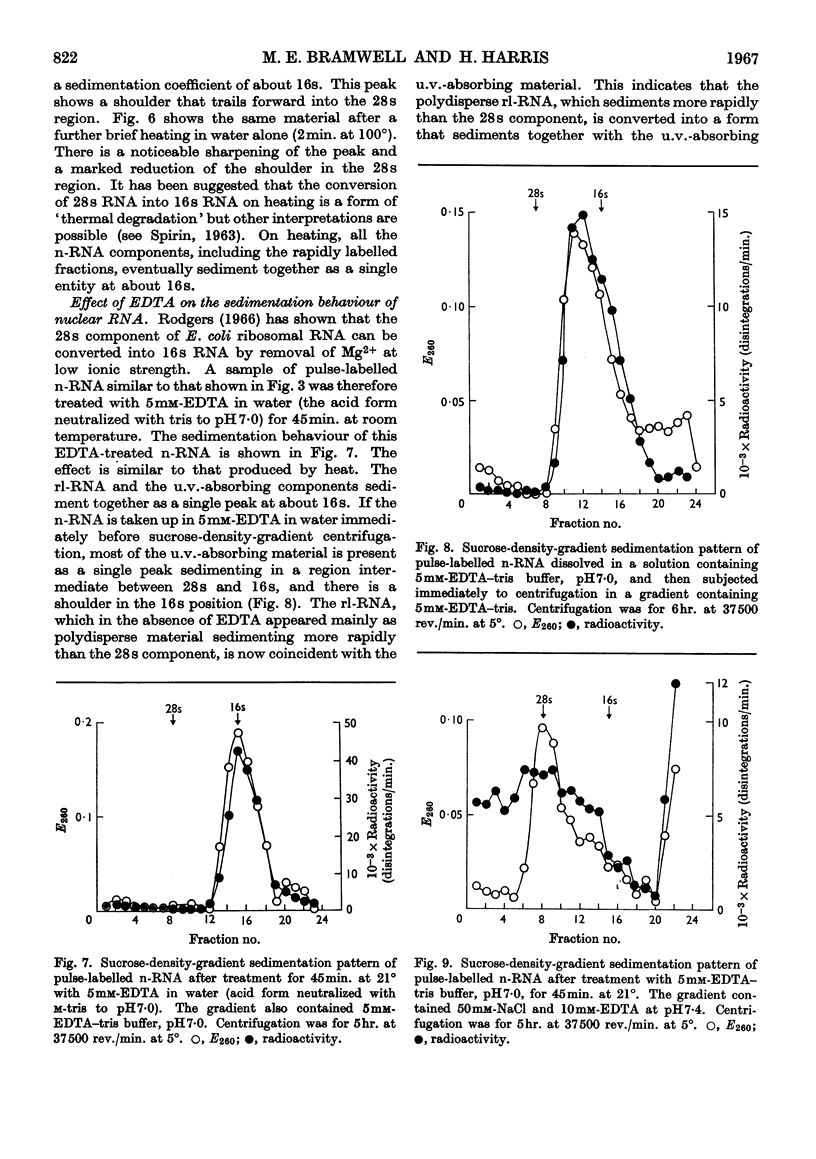
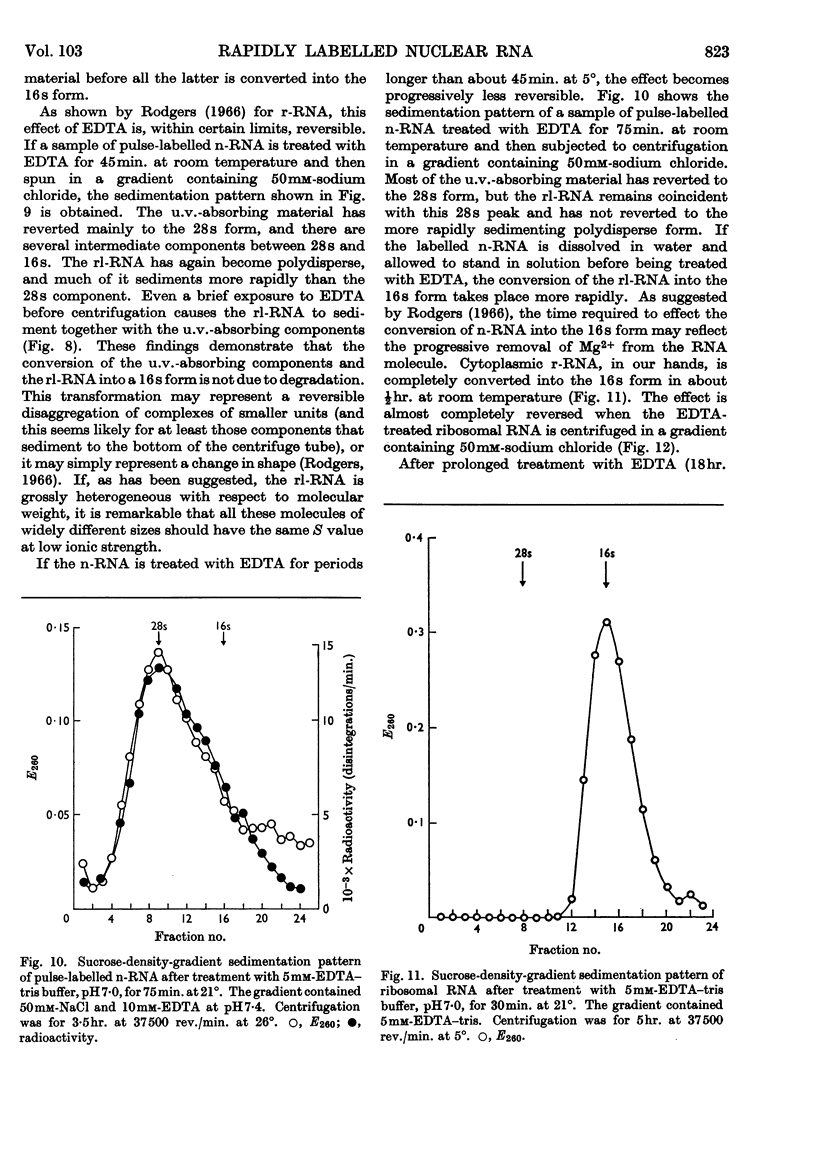
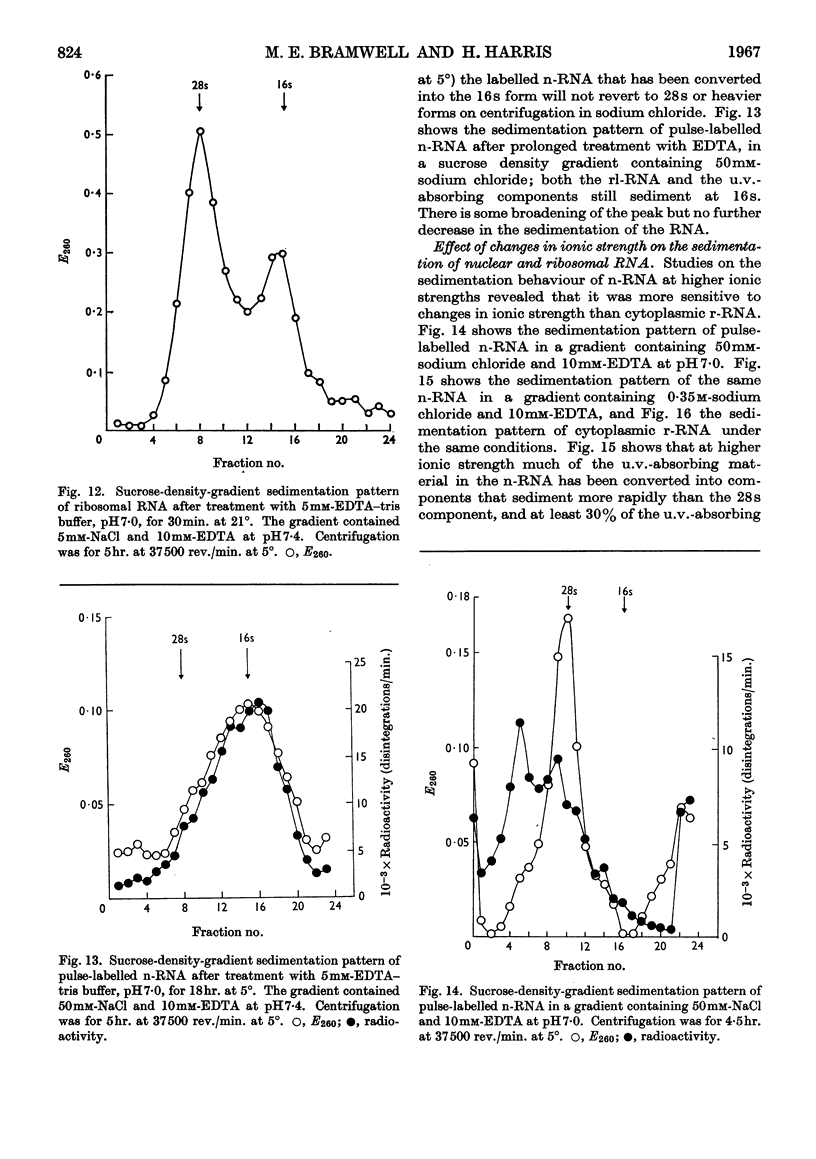
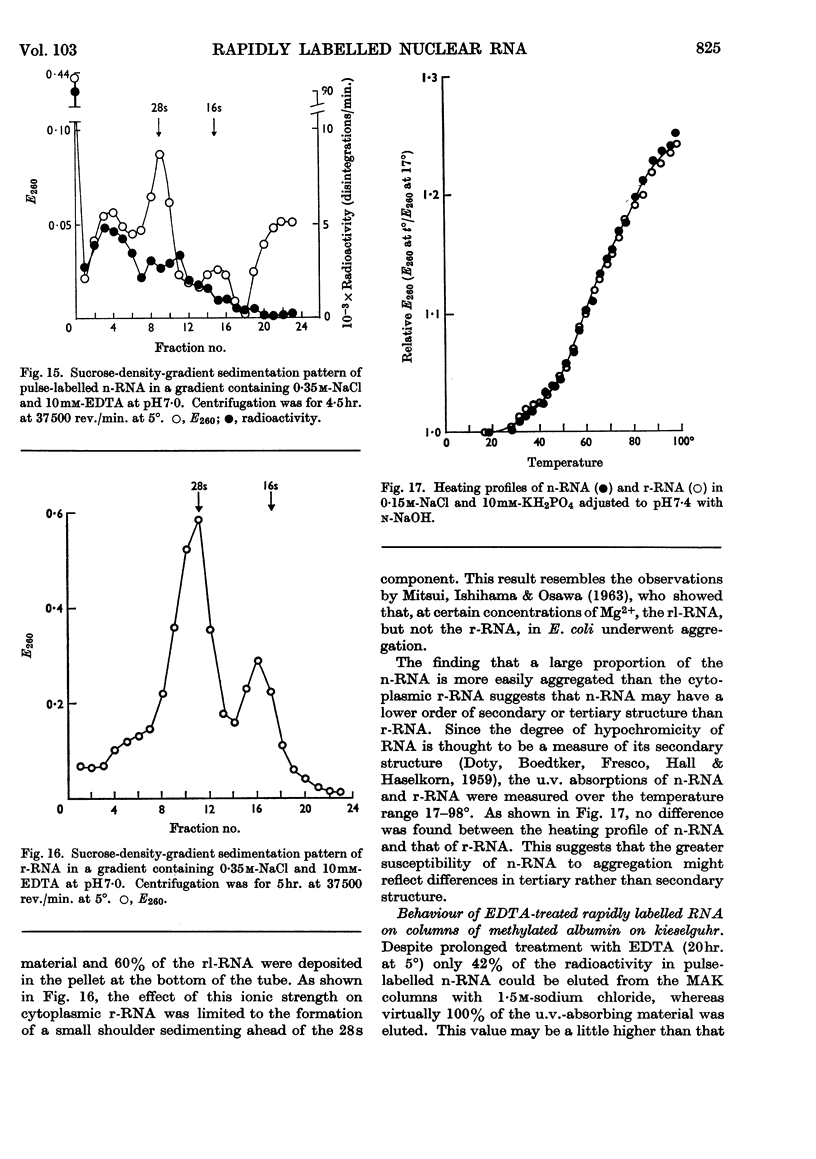
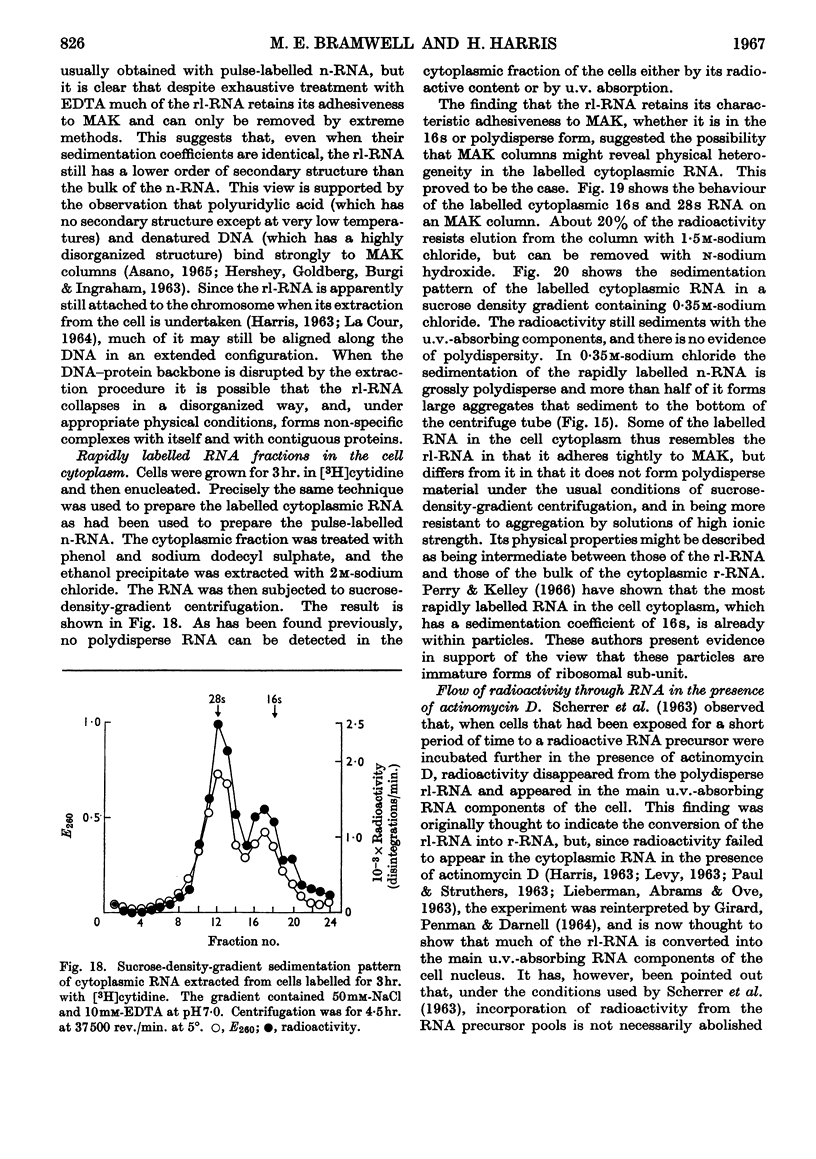
1. A study was made of the sedimentation properties of purified preparations of the rapidly labelled RNA in the nucleus and the cytoplasm of the HeLa cell. The sedimentation of the rapidly labelled nuclear RNA was very sensitive to changes in ionic strength and bivalent cation concentration. Under the conditions usually used in sucrose-density-gradient centrifugation the rapidly labelled nuclear RNA showed extreme polydispersity, and much of it sedimented more rapidly than the 28s RNA. At low ionic strength and after removal of Mg2+, however, the rapidly labelled nuclear RNA sedimented as a single peak at about 16s. The conversion of the polydisperse material into the 16s form did not involve degradation of the RNA, since the effect could be reversed by increasing the ionic strength of the solution. 2. The cytoplasm did not contain any RNA that showed polydisperse sedimentation under the usual conditions of sucrose-density-gradient centrifugation, or that had the same sensitivity as the rapidly labelled nuclear RNA to changes in ionic strength. All the radioactivity in the cytoplasmic RNA sedimented with the 28s, 16s and 4s components over a wide range of physical conditions, but these components did contain a labelled fraction with some of the features of the rapidly labelled nuclear RNA on columns of methylated albumin on kieselguhr. 3. In both nucleus and cytoplasm the RNA detected by ultraviolet absorption could also be converted into a 16s form by removal of bivalent cations at low ionic strength; this effect was again, within certain limits, reversible. The nuclear RNA as a whole was more susceptible to changes in ionic strength than the cytoplasmic RNA. 4. It thus appears that all the RNA in the cell, except the 4s RNA, can be prepared, without degradation, as a single peak sedimenting at about 16s. The relationship of these various 16s components to each other is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H. The relationship between cellular nucleic acids in the developing rat cerebral cortex. Biochem J. 1966 Feb;98(2):636–640. doi: 10.1042/bj0980636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi G., Parnas H., Hwang M. I., Attardi B. Giant-size rapidly labeled nuclear ribonucleic acid and cytoplasmic messenger ribonucleic acid in immature duck erythrocytes. J Mol Biol. 1966 Sep;20(1):145–182. doi: 10.1016/0022-2836(66)90123-9. [DOI] [PubMed] [Google Scholar]
- BRUNS G. P., FISCHER S., LOWY B. A. A STUDY OF THE SYNTHESIS AND INTERRELATIONSHIPS OF RIBONUCLEIC ACIDS IN DUCK ERYTHROCYTES. Biochim Biophys Acta. 1965 Feb 8;95:280–290. doi: 10.1016/0005-2787(65)90492-2. [DOI] [PubMed] [Google Scholar]
- Bellamy A. R. RNA synthesis in exponentially growing tobacco cells subjected to a step-down nutritional shift. Biochim Biophys Acta. 1966 Jul 20;123(1):102–115. doi: 10.1016/0005-2787(66)90163-8. [DOI] [PubMed] [Google Scholar]
- Bishop D. H. Physical properties of single- and double-stranded coliphage ribonucleic acid. Biochem J. 1966 Aug;100(2):321–329. doi: 10.1042/bj1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWLEY J. C., HARRIS H. THE FINE STRUCTURE OF ISOLATED HELA CELL NUCLEI. Exp Cell Res. 1963 Jun;31:70–81. doi: 10.1016/0014-4827(63)90156-3. [DOI] [PubMed] [Google Scholar]
- DOTY P., BOEDTKER H., FRESCO J. R., HALL B. D., HASELKORN R. Configurational studies of polynucleotides and ribonucleic acid. Ann N Y Acad Sci. 1959 Sep 4;81:693–708. doi: 10.1111/j.1749-6632.1959.tb49351.x. [DOI] [PubMed] [Google Scholar]
- Denborough M. A., Ogston A. G. Failure of phenol to remove residual protein from hyaluronic acid. Nature. 1965 Sep 25;207(5004):1389–1389. doi: 10.1038/2071389a0. [DOI] [PubMed] [Google Scholar]
- EAGLE H., OYAMA V. I., LEVY M., HORTON C. L., FLEISCHMAN R. The growth response of mammalian cells in tissue culture to L-glutamine and L-glutamic acid. J Biol Chem. 1956 Feb;218(2):607–616. [PubMed] [Google Scholar]
- Ellem K. A., Sheridan J. W. Tenacious binding of the bulk of the DNA-like RNA of metazoan cells to methylated albumin columns. Biochem Biophys Res Commun. 1964 Aug 11;16(6):505–510. doi: 10.1016/0006-291x(64)90183-4. [DOI] [PubMed] [Google Scholar]
- FENWICK M. L. THE FATE OF RAPIDLY LABELLED RIBONUCLEIC ACID IN THE PRESENCE OF ACTINOMYCIN IN NORMAL AND VIRUS-INFECTED ANIMAL CELLS. Biochim Biophys Acta. 1964 Jul 22;87:388–396. doi: 10.1016/0926-6550(64)90112-4. [DOI] [PubMed] [Google Scholar]
- GIRARD M., PENMAN S., DARNELL J. E. THE EFFECT OF ACTINOMYCIN ON RIBOSOME FORMATION IN HELA CELLS. Proc Natl Acad Sci U S A. 1964 Feb;51:205–211. doi: 10.1073/pnas.51.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROS F., HIATT H., GILBERT W., KURLAND C. G., RISEBROUGH R. W., WATSON J. D. Unstable ribonucleic acid revealed by pulse labelling of Escherichia coli. Nature. 1961 May 13;190:581–585. doi: 10.1038/190581a0. [DOI] [PubMed] [Google Scholar]
- HARRIS H. FUNCTION OF THE SHORT-LIVED RIBONUCLEIC ACID IN THE CELL NUCLEUS. Nature. 1964 Feb 29;201:863–867. doi: 10.1038/201863a0. [DOI] [PubMed] [Google Scholar]
- HARRIS H. Rapidly labelled ribonucleic acid in the cell nucleus. Nature. 1963 Apr 13;198:184–185. doi: 10.1038/198184a0. [DOI] [PubMed] [Google Scholar]
- HARRIS H. Turnover of nuclear and cytoplasmic ribonucleic acid in two types of animal cell, with some further observations on the nucleolus. Biochem J. 1959 Oct;73:362–369. doi: 10.1042/bj0730362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS H., WATTS J. W. The relationship between nuclear and cytoplasmic ribonucleic acid. Proc R Soc Lond B Biol Sci. 1962 May 15;156:109–121. doi: 10.1098/rspb.1962.0031. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., GOLDBERG E., BURGI E., INGRAHAM L. Local denaturation of DNA by shearing forces and by heat. J Mol Biol. 1963 Mar;6:230–243. doi: 10.1016/s0022-2836(63)80072-8. [DOI] [PubMed] [Google Scholar]
- HIATT H. H. A rapidly labeled RNA in rat liver nuclei. J Mol Biol. 1962 Aug;5:217–229. doi: 10.1016/s0022-2836(62)80085-0. [DOI] [PubMed] [Google Scholar]
- Houssais J. F., Attardi G. High molecular weight nonribosomal-type nuclear RNA and cytoplasmic messenger RNA in HeLa cells. Proc Natl Acad Sci U S A. 1966 Aug;56(2):616–623. doi: 10.1073/pnas.56.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIHAMA A., MIZUNO N., TAKAI M., OTAKA E., OSAWA S. Molecular and metabolic properties of messenger RNA from normal and T2-infected Escherichia coli. J Mol Biol. 1962 Sep;5:251–264. doi: 10.1016/s0022-2836(62)80069-2. [DOI] [PubMed] [Google Scholar]
- KIDSON C., KIRBY K. S., RALPH R. K. ISOLATION CHARACTERISTICS OF RAPIDLY LABELLED RNA FROM NORMAL RAT LIVER. J Mol Biol. 1963 Sep;7:312–315. doi: 10.1016/s0022-2836(63)80010-8. [DOI] [PubMed] [Google Scholar]
- KITAZUME Y., YCAS M., VINCENT W. S. Metabolic properties of a ribonucleic acid fraction in yeast. Proc Natl Acad Sci U S A. 1962 Feb;48:265–282. doi: 10.1073/pnas.48.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubinski H., Koch G. Regulation of the synthesis of various ribonucleic acids in animal cells. Biochem Biophys Res Commun. 1966 Feb 3;22(3):346–351. doi: 10.1016/0006-291x(66)90489-x. [DOI] [PubMed] [Google Scholar]
- LACOUR L. F. BEHAVIOUR OF NUCLEOLI IN ISOLATED NUCLEI. Exp Cell Res. 1964 Apr;34:239–242. doi: 10.1016/0014-4827(64)90360-x. [DOI] [PubMed] [Google Scholar]
- LEVY H. B. EFFECT OF ACTINOMYCIN D ON HELA CELL NUCLEAR RNA METABOLISM. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:886–889. doi: 10.3181/00379727-113-28521. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN I., ABRAMS R., OVE P. Changes in the metabolism of ribonucleic acid preceding the synthesis of deoxyribonucleic acid in mammalian cells cultured from the animal. J Biol Chem. 1963 Jun;238:2141–2149. [PubMed] [Google Scholar]
- Ludlum D. B. Methylation and secondary structure of polyadenylic acid. Biochim Biophys Acta. 1966 Jun 22;119(3):630–632. doi: 10.1016/0005-2787(66)90140-7. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- MARTINEZSEGOVIA Z. M., SOKOL F., GRAVES I. L., ACKERMANN W. W. SOME PROPERTIES OF NUCLEIC ACIDS EXTRACTED WITH PHENOL. Biochim Biophys Acta. 1965 Feb 8;95:329–340. doi: 10.1016/0005-2787(65)90497-1. [DOI] [PubMed] [Google Scholar]
- MIDGLEY J. E., McCARTHY B. J. The synthesis and kinetic behavior of deoxyribonucleic acid-like ribonucleic acid in bacteria. Biochim Biophys Acta. 1962 Nov 26;61:696–717. doi: 10.1016/0926-6550(62)90053-1. [DOI] [PubMed] [Google Scholar]
- MITSUI H., ISHIHAMA A., OSAWA S. SOME PROPERTIES OF NEWLY SYNTHESIZED RIBOSOMAL RIBONUCLEIC ACID IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Nov 22;76:401–409. [PubMed] [Google Scholar]
- MONIER R., NAONO S., HAYES D., HAYES F., GROS F. Studies on the heterogeneity of messenger RNA from E. Coli. J Mol Biol. 1962 Sep;5:311–324. doi: 10.1016/s0022-2836(62)80075-8. [DOI] [PubMed] [Google Scholar]
- Marbaix G., Burny A., Huez G., Chantrenne H. Base composition of messenger RNA from rabbit reticulocytes. Biochim Biophys Acta. 1966 Feb 21;114(2):404–406. doi: 10.1016/0005-2787(66)90320-0. [DOI] [PubMed] [Google Scholar]
- Owen M. Uptake of [3H] uridine into precursor pools and RNA in osteogenic cells. J Cell Sci. 1967 Mar;2(1):39–56. doi: 10.1242/jcs.2.1.39. [DOI] [PubMed] [Google Scholar]
- PAUL J., STRUTHERS M. G. Actinomycin D-resistant RNA synthesis in animal cells. Biochem Biophys Res Commun. 1963 Apr 23;11:135–139. doi: 10.1016/0006-291x(63)90080-9. [DOI] [PubMed] [Google Scholar]
- PETERMANN M. L., PAVLOVEC A. STUDIES ON RIBONUCLEIC ACID FROM RAT LIVER RIBOSOMES. J Biol Chem. 1963 Nov;238:3717–3724. [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Buoyant densities of cytoplasmic ribonucleoprotein particles of mammalian cells: distinctive character of ribosome subunits and the rapidly labeled components. J Mol Biol. 1966 Apr;16(2):255–268. doi: 10.1016/s0022-2836(66)80171-7. [DOI] [PubMed] [Google Scholar]
- Perry R. P. THE CELLULAR SITES OF SYNTHESIS OF RIBOSOMAL AND 4S RNA. Proc Natl Acad Sci U S A. 1962 Dec;48(12):2179–2186. doi: 10.1073/pnas.48.12.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontecorvo G. Template and stepwise processes in heredity. Proc R Soc Lond B Biol Sci. 1966 Mar 22;164(995):167–169. doi: 10.1098/rspb.1966.0020. [DOI] [PubMed] [Google Scholar]
- RAKE A. V., GRAHAM A. F. KINETICS OF INCORPORATION OF URIDINE-C14 INTO L CELL RNA. Biophys J. 1964 Jul;4:267–284. doi: 10.1016/s0006-3495(64)86782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K. Studies on RNA synthesis in Ehrlich ascites cells extraction and properties of labeled RNA. Biochim Biophys Acta. 1965 Nov 8;108(3):474–488. doi: 10.1016/0005-2787(65)90039-0. [DOI] [PubMed] [Google Scholar]
- Rodgers A. Magnesium ions and the structure of Escherichia coli ribosomal ribonucleic acid. Biochem J. 1966 Jul;100(1):102–109. doi: 10.1042/bj1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., LATHAM H., DARNELL J. E. Demonstration of an unstable RNA and of a precursor to ribosomal RNA in HeLa cells. Proc Natl Acad Sci U S A. 1963 Feb 15;49:240–248. doi: 10.1073/pnas.49.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saponara A. G., Enger M. D. Incorporation of [3-H]uridine and [M3-14-C]methionine into Chinese-hamster cell ribonucleic acid. Biochim Biophys Acta. 1966 Jun 22;119(3):492–500. [PubMed] [Google Scholar]
- Soeiro R., Birnboim H. C., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. II. Base composition and cellular localization of a heterogeneous RNA fraction. J Mol Biol. 1966 Aug;19(2):362–372. doi: 10.1016/s0022-2836(66)80010-4. [DOI] [PubMed] [Google Scholar]
- TAKAI M., KONDO N., OSAWA S. Ribonucleic acid having deoxyribonucleic acid-type nucleotide composition in Escherichia coli. Biochim Biophys Acta. 1962 Mar 5;55:416–418. doi: 10.1016/0006-3002(62)90807-7. [DOI] [PubMed] [Google Scholar]
- Tsanev R. G., Markov G. G., Dessev G. N. Incorporation of labelled precursors into the electrophoretic fractions of rat-liver ribonucleic acid. Biochem J. 1966 Jul;100(1):204–210. doi: 10.1042/bj1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLKIN E., ASTRACHAN L., COUNTRYMAN J. L. Metabolism of RNA phosphorus in Escherichia coli infected with bacteriophage T7. Virology. 1958 Oct;6(2):545–555. doi: 10.1016/0042-6822(58)90101-6. [DOI] [PubMed] [Google Scholar]
- WATTS J. W., HARRIS H. Turnover of nucleic acids in a non-multiplying animal cell. Biochem J. 1959 May;72(1):147–153. doi: 10.1042/bj0720147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]
- Watts J. W. Turnover of nucleic acids in a multiplying animal cell. 2. Retention studies. Biochem J. 1964 Nov;93(2):306–312. doi: 10.1042/bj0930306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Fukada T., Kawade Y. Separation of rapidly labeled RNA of animal cells into DNA-type and ribosomal RNA-type components. Biochem Biophys Res Commun. 1964 Feb 18;15(1):22–26. doi: 10.1016/0006-291x(64)90096-8. [DOI] [PubMed] [Google Scholar]


