Abstract
The incorporation of 14C by etiolated maize and barley shoots exposed to light of 14CO2 and [2-14C]mevalonic acid into phylloquinone, plastoquinone, ubiquinone, α-tocopherolquinone and α-tocopherol was examined. In maize (the principal tissue studied) it was demonstrated that 14C from [2-14C]mevalonic acid is incorporated into phylloquinone, plastoquinone and ubiquinone. α-Tocopherol and α-tocopherolquinone, although undoubtedly labelled from this substrate, were not purified completely. As expected, 14C from 14CO2 was incorporated into all components examined. Ozonolytic degradation studies showed that 14C from [2-14C]mevalonic acid was incorporated specifically into the prenyl side chains of plastoquinone and ubiquinone, and from this it was inferred that mevalonic acid can be regarded as the specific distal precursor to the prenyl portions of all terpenoid quinones occurring in plant tissues. From a comparison of the relative incorporation of 14C from 14CO2 and [2-14C]mevalonic acid into the intra- and extra-chloroplastidic terpenoids evidence was obtained consistent with the tenet that the prenyl portions of the chloroplastidic quinones phylloquinone and plastoquinone, along with β-carotene, are biosynthesized within the confines of the chloroplast, the side chain of the extraplastidic ubiquinone and phytosterols being synthesized elsewhere within the cell. The results obtained for the incorporation of 14C from 14CO2 and [2-14C]mevalonic acid into α-tocopherol and α-tocopherolquinone were not readily interpretable with regard to the site of synthesis of these compounds.
Full text
PDF




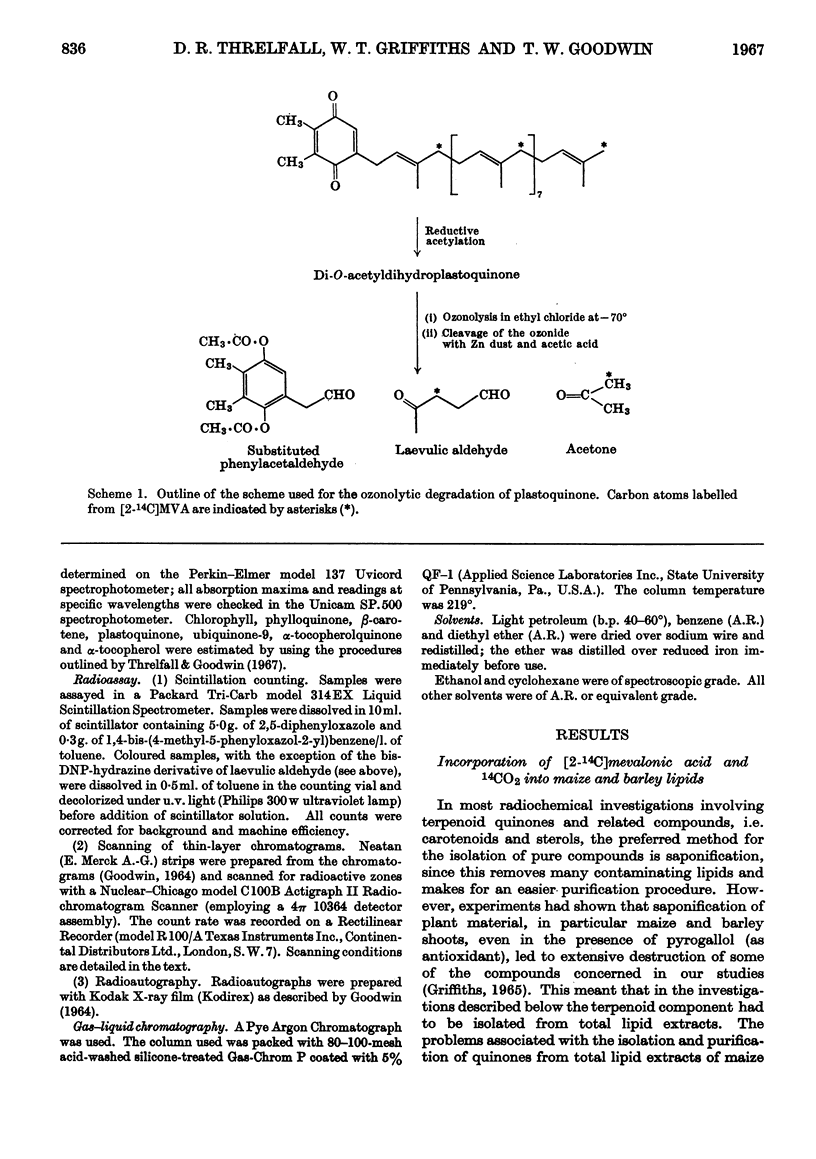
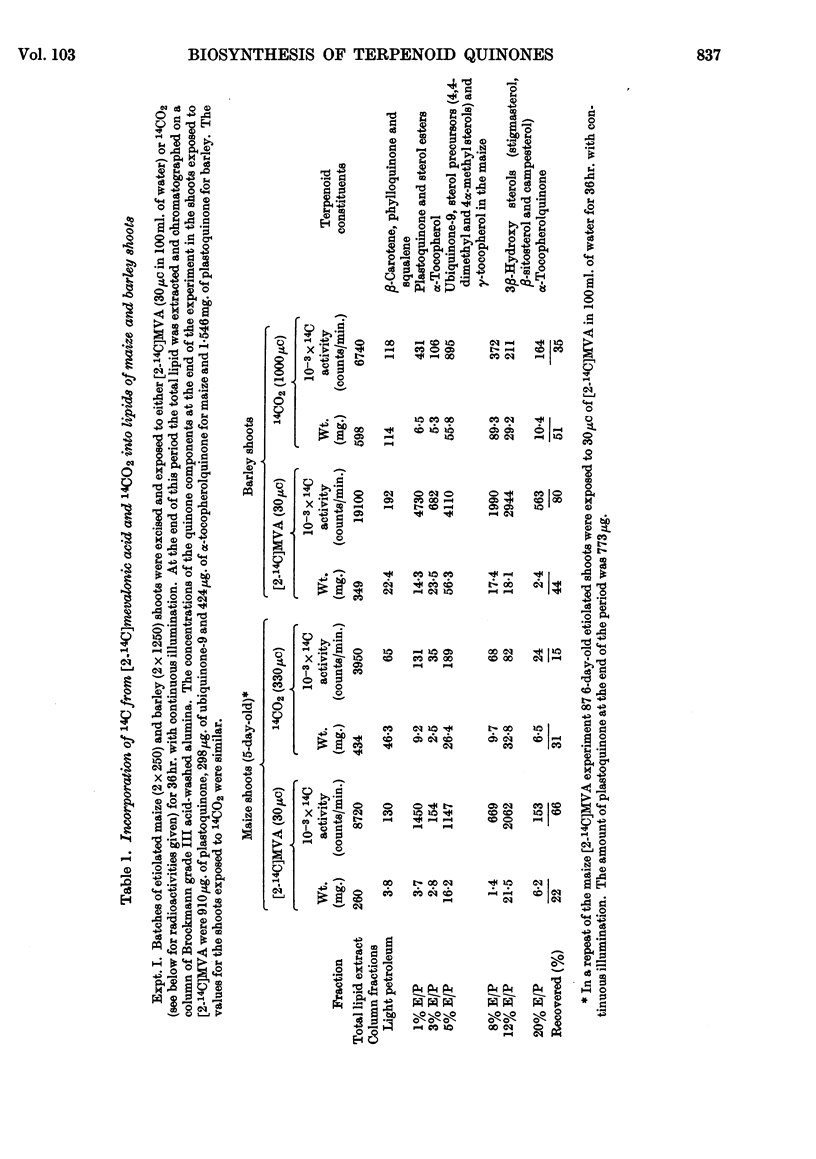

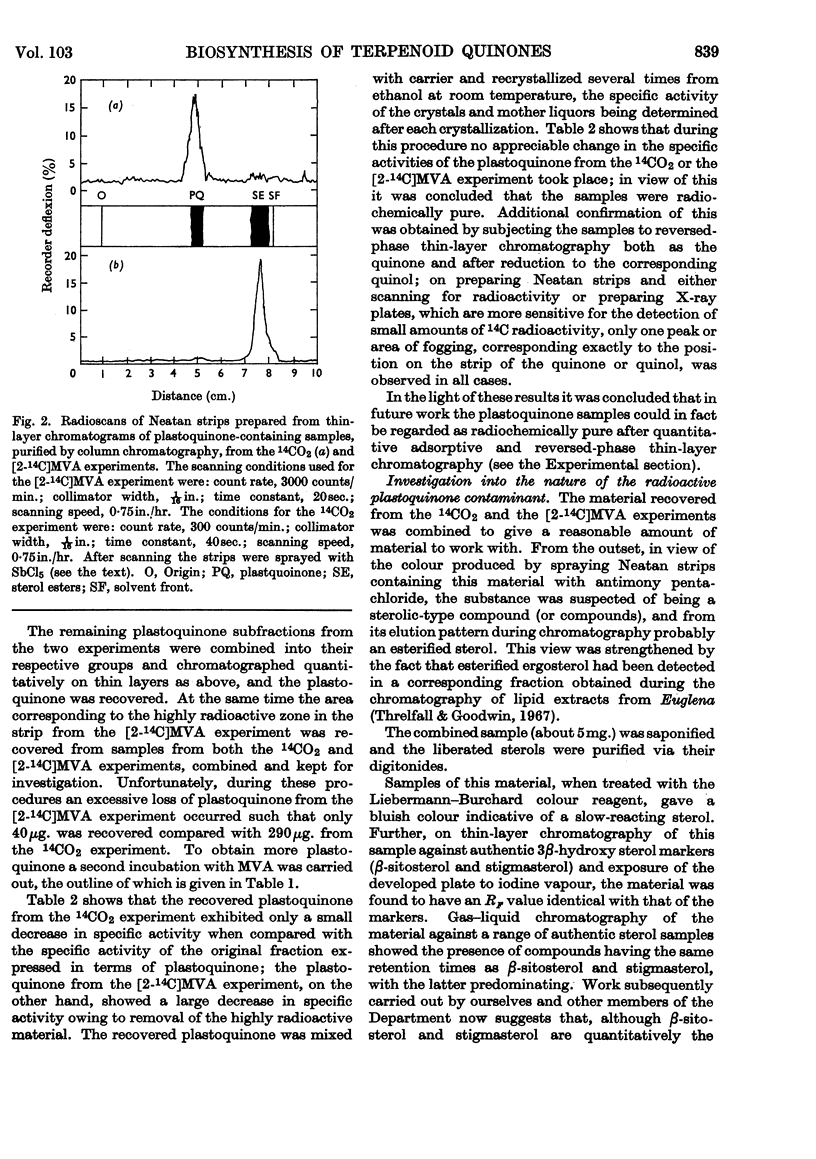
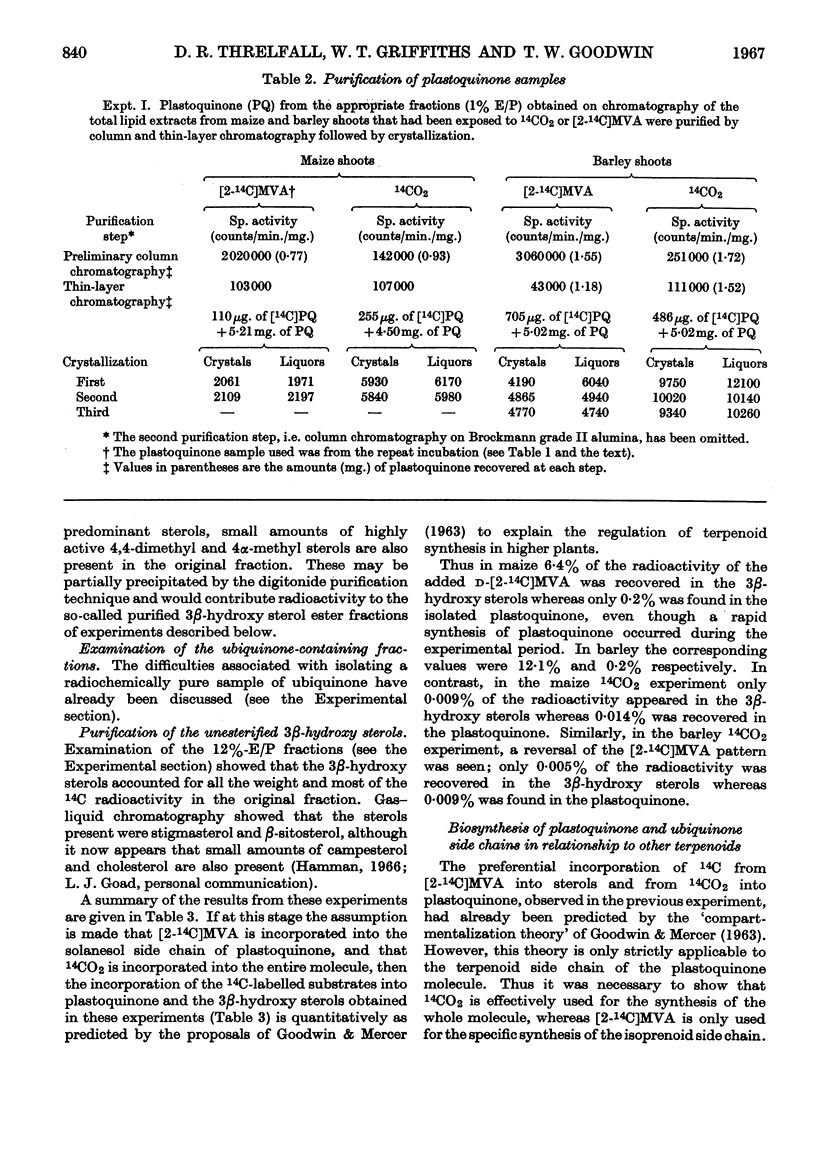








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGOS J., HEMMING F. W., PENNOCK J. F., MORTON R. A. DOLICHOL: A NATURALLY-OCCURRING C100 ISOPRENOID ALCOHOL. Biochem J. 1963 Sep;88:470–482. [PMC free article] [PubMed] [Google Scholar]
- DILLEY R. A., CRANE F. L. A specific assay for tocopherols in plant tissue. Anal Biochem. 1963 Jun;5:531–541. doi: 10.1016/0003-2697(63)90073-3. [DOI] [PubMed] [Google Scholar]
- DILLEY R. A. THIN-LAYER CHROMATOGRAPHY OF NATURALLY OCCURRING QUINONES AND HYDROQUINONES. Anal Biochem. 1964 Feb;7:240–246. doi: 10.1016/0003-2697(64)90234-9. [DOI] [PubMed] [Google Scholar]
- GOODWIN T. W. Studies in carotenogenesis. 25. The incorporation of 14CO2, [2-14C] acetate and [2-14C]mevalonate into beta-carotene by illuminated etiolated maize seedings. Biochem J. 1958 Dec;70(4):612–617. doi: 10.1042/bj0700612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad L. J., Goodwin T. W. The biosynthesis of sterols in higher plants. Biochem J. 1966 Jun;99(3):735–746. doi: 10.1042/bj0990735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths W. T., Threlfall D. R., Goodwin T. W. Nature, intracellular distribution and formation of terpenoid quinones in maize and barley shoots. Biochem J. 1967 May;103(2):589–600. doi: 10.1042/bj1030589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSEN R. K., SMITH J. L., DAVES G. D., MOORE H. W., FOLKERS K., PARSON W. W., RUDNEY H. 2-DECAPRENYLPHENOL, BIOSYNTHETIC PRECURSOR OF UBIQUINONE-10. J Am Chem Soc. 1965 May 20;87:2298–2300. doi: 10.1021/ja01088a045. [DOI] [PubMed] [Google Scholar]
- PARSON W. W., RUDNEY H. THE BIOSYNTHESIS OF THE BENZOQUINONE RING OF UBIQUINONE FROM P-HYDROXYBENZALDEHYDE AND P-HYDROXYBENZOIC ACID IN RAT KIDNEY, AZOTOBACTER VINELANDII, AND BAKER'S YEAST. Proc Natl Acad Sci U S A. 1964 Mar;51:444–450. doi: 10.1073/pnas.51.3.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennock J. F., Hemming F. W., Kerr J. D. A reassessment of tocopherol in chemistry. Biochem Biophys Res Commun. 1964 Nov 30;17(5):542–548. doi: 10.1016/0006-291x(64)90062-2. [DOI] [PubMed] [Google Scholar]
- Rogers L. J., Shah S. P., Goodwin T. W. Intracellular localization of mevalonate-activating enzymes in plant cells. Biochem J. 1966 May;99(2):381–388. doi: 10.1042/bj0990381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall D. R., Goodwin T. W. Nature, intracellular distribution and formation of terpenoid quinones in Euglena gracilis. Biochem J. 1967 May;103(2):573–588. doi: 10.1042/bj1030573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treharne K. J., Mercer E. I., Goodwin T. W. Incorporation of [14C] carbon dioxide and [2-14C] mevalonic acid into terpenoids of higher plants during chloroplast development. Biochem J. 1966 Apr;99(1):239–245. doi: 10.1042/bj0990239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R., Goodwin T. W. Incorporation of [G-14cC]shikimate and [U-14C]para-hydroxybenzoate into phytoquinones and chromanols. Biochem Biophys Res Commun. 1966 Jun 21;23(6):849–853. doi: 10.1016/0006-291x(66)90565-1. [DOI] [PubMed] [Google Scholar]


