Abstract
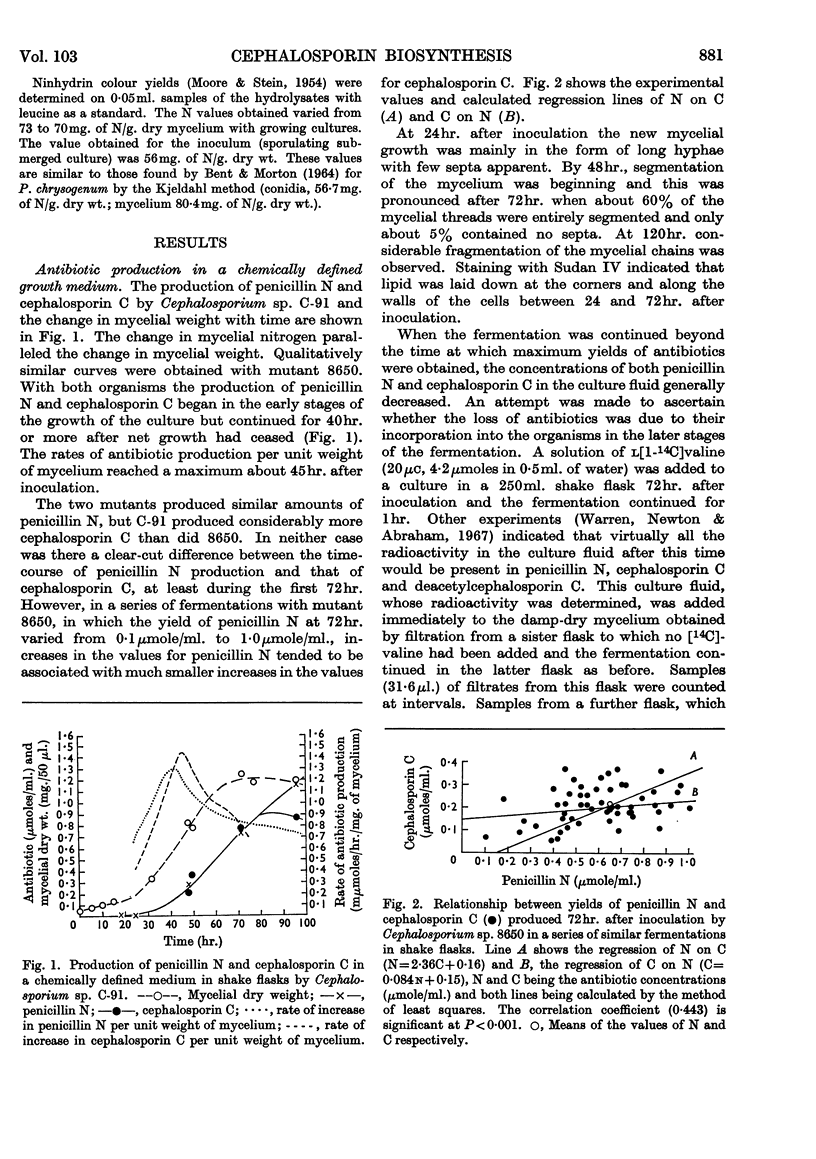
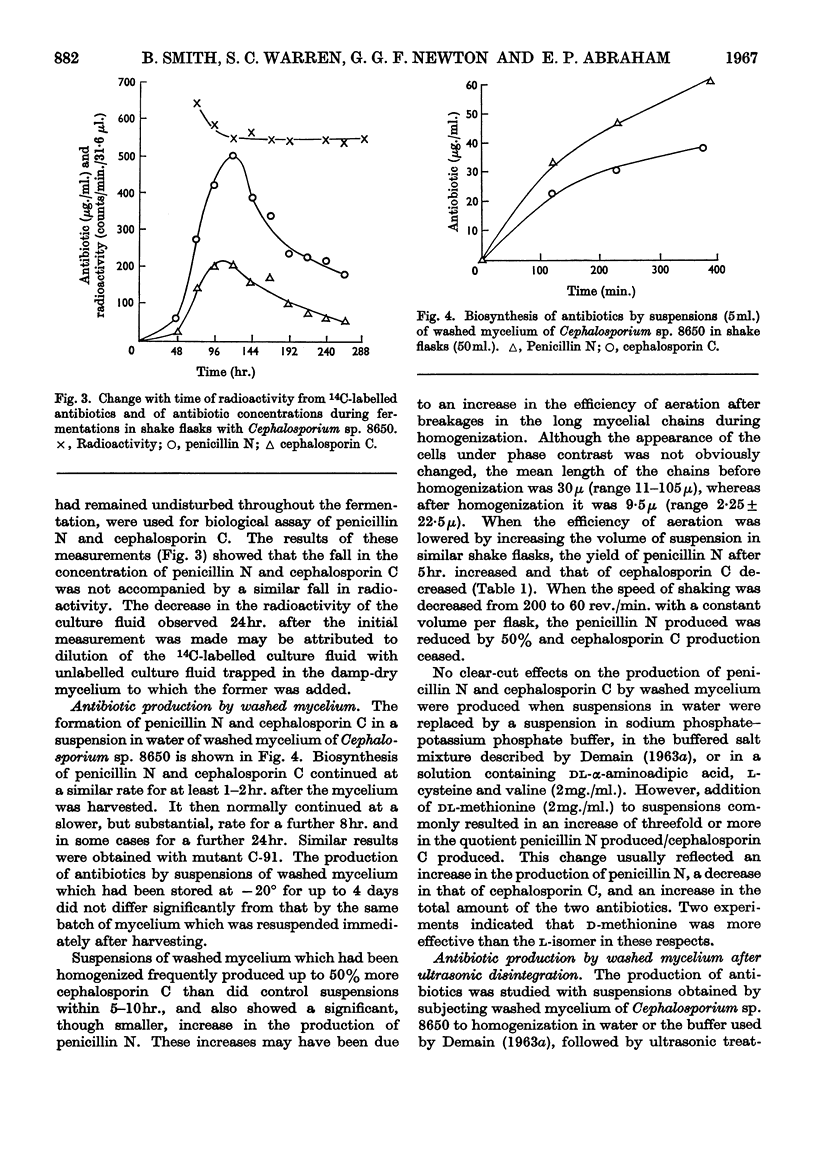
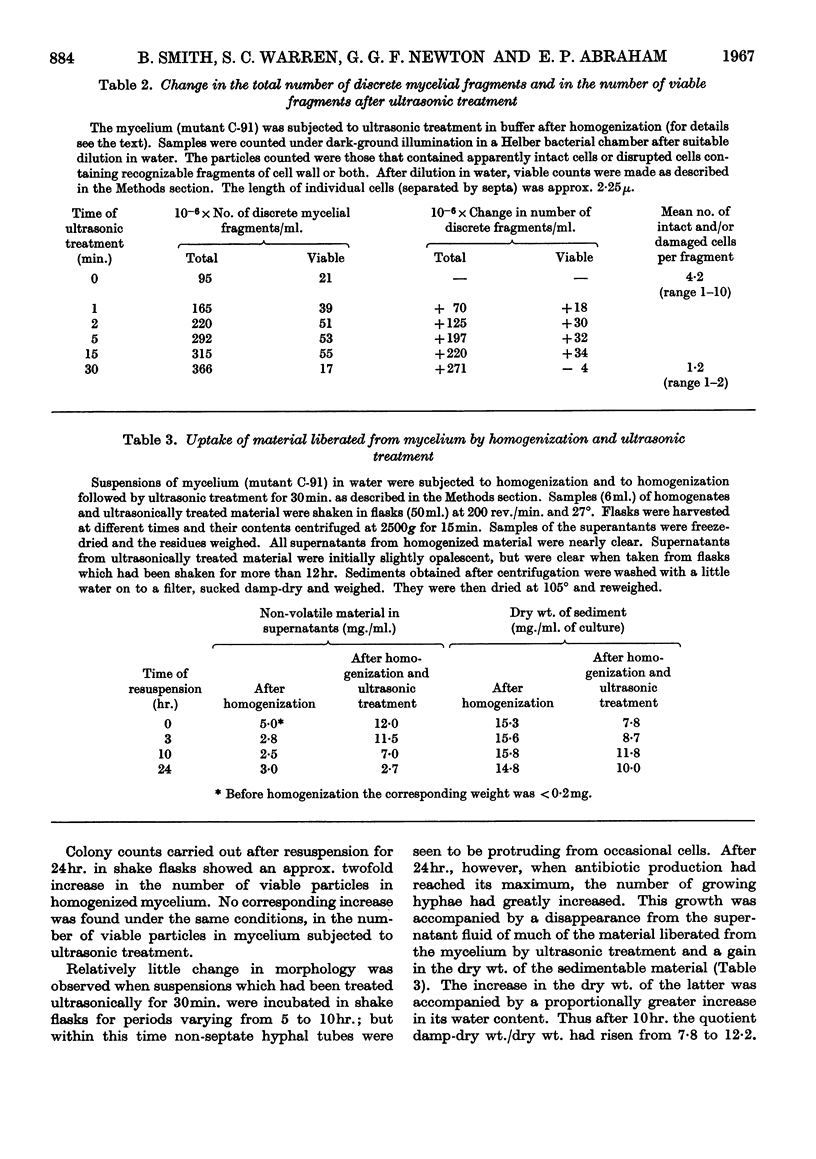
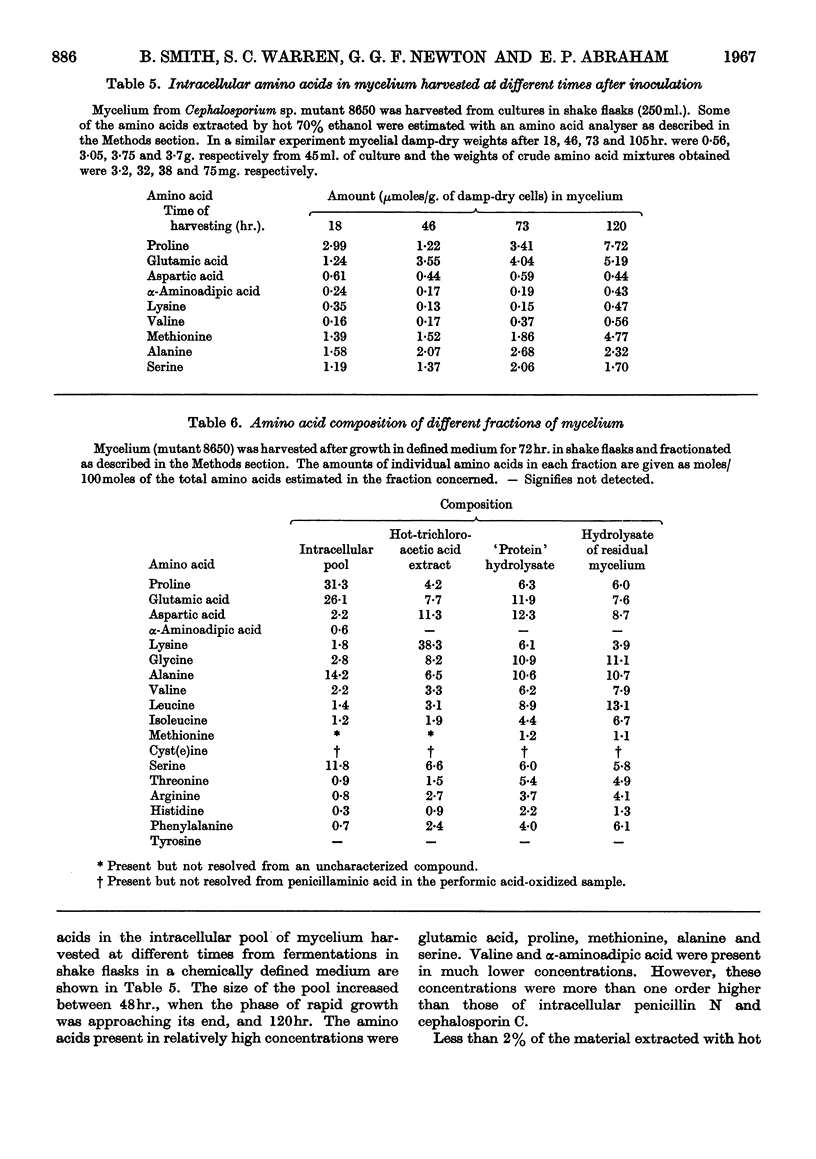
1. The production of penicillin N and cephalosporin C by two mutants of a Cephalosporium sp. has been studied with cultures grown in a chemically defined medium and with suspensions of washed mycelium in water or a buffered salt solution. 2. Antibiotic synthesis began at an early stage of growth and its rate per unit weight of mycelium appeared to pass its maximum as morphological changes were occurring in young hyphae. This rate subsequently declined, but rapid production could continue after net growth had ceased. 3. In a series of shake-flask fermentations in the growth medium, increases in the yield of penicillin N above the mean were correlated with much smaller increases in the yield of cephalosporin C and vice versa. 4. In suspensions of washed mycelium, moderate decreases in the efficiency of aeration increased the yield of penicillin N and decreased that of cephalosporin C. A similar result normally followed the addition of methionine to the suspension fluid, and in both cases there was usually an increase in the yield of the two antibiotics combined. 5. The apparent intracellular concentrations of the antibiotics were much lower than those attained extracellularly and also much lower than those of most of the amino acids in the intracellular pool. No detectable amount of [14C]penicillin N added to the extracellular fluid was found to enter the mycelium. 6. Very small amounts of peptide material whose behaviour was similar to that of the sulphonic acid of δ-(α-amino-adipoyl)cysteinylvaline on paper electrophoresis at pH1·8 were found in extracts of the mycelium that had been oxidized with performic acid. 6-Aminopenicillanic acid and 7-aminocephalosporanic acid were not detected. 7. Ultrasonic treatment of the mycelium resulted in rapid fragmentation of mycelial chains, rupture of many individual cells, and the liberation of amino acids and other substances into the medium. 8. Ultrasonically treated preparations synthesized penicillin N and cephalosporin C rapidly after a lag of 12hr. Antibiotic synthesis was accompanied by the growth of hyphae from swollen mycelial fragments and by the re-establishment of permeability barriers resulting in the uptake of amino acids from the medium.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM E. P., NEWTON G. G. The structure of cephalesporin C. Biochem J. 1961 May;79:377–393. doi: 10.1042/bj0790377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARNSTEIN H. R., ARTMAN M., MORRIS D., TOMS E. J. Sulphur-containing amino acids and peptides in the mycelium of Penicillium chrysogenum. Biochem J. 1960 Aug;76:353–357. doi: 10.1042/bj0760353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON H. S., ABRAHAM E. P. Isolation of antibiotics from a species of Cephalosporium; cephalosporins P1, P2, P3, P4, and P5. Biochem J. 1951 Dec;50(2):168–174. doi: 10.1042/bj0500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bent K. J., Morton A. G. Amino acid composition of fungi during development in submerged culture. Biochem J. 1964 Aug;92(2):260–269. doi: 10.1042/bj0920260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMAIN A. L., NEWKIRK J. F., HENDLIN D. Effect of methionine, norleucine, and lysine derivatives on cephalosporin C formation in chemically defined media. J Bacteriol. 1963 Feb;85:339–344. doi: 10.1128/jb.85.2.339-344.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMAIN A. L. SYNTHESIS OF CEPHALOSPORIN C BY RESTING CELLS OF CEPHALOSPORIUM SP. Clin Med (Northfield) 1963 Nov;70:2045–2051. [PubMed] [Google Scholar]
- FLYNN F. V., DE MAYO P. Microelectrophoresis of protein on filter-paper. Lancet. 1951 Aug 11;2(6676):235–239. doi: 10.1016/s0140-6736(51)93239-4. [DOI] [PubMed] [Google Scholar]
- JONES E. E., BROQUIST H. P. SACCHAROPINE, AN INTERMEDIATE OF THE AMINOADIPIC ACID PATHWAY OF LYSINE BIOSYNTHESIS. II. STUDIES IN SACCHAROMYCES CEREVISEAE. J Biol Chem. 1965 Jun;240:2531–2536. [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- KAVANAGH F., TUNIN D., WILD G. D-methionine and the biosynthesis of cephalosporin N. Arch Biochem Biophys. 1958 Oct;77(2):268–274. doi: 10.1016/0003-9861(58)90075-4. [DOI] [PubMed] [Google Scholar]
- LODER B., NEWTON G. G., ABRAHAM E. P. The cephalosporin C nucleus (7-aminocephalosporanic acid) and some of its derivatives. Biochem J. 1961 May;79:408–416. doi: 10.1042/bj0790408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J Biol Chem. 1954 Dec;211(2):907–913. [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. Chromatography of amino acids on sulfonated polystyrene resins. J Biol Chem. 1951 Oct;192(2):663–681. [PubMed] [Google Scholar]
- Ott J. L., Godzeski C. W., Pavey D., Farran J. D., Horton D. R. Biosynthesis of Cephalosporin C: I. Factors Affecting the Fermentation. Appl Microbiol. 1962 Nov;10(6):515–523. doi: 10.1128/am.10.6.515-523.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWBURY R. J., WOODS D. D. REPRESSION BY METHIONINE OF CYSTATHIONASE FORMATION IN ESCHERICHIA COLI. J Gen Microbiol. 1964 Apr;35:145–158. doi: 10.1099/00221287-35-1-145. [DOI] [PubMed] [Google Scholar]
- Snoke J. E. Amino acid composition of Bacillus licheniformis. Biochem Biophys Res Commun. 1964;14:571–574. doi: 10.1016/0006-291x(64)90271-2. [DOI] [PubMed] [Google Scholar]
- TROWN P. W., ABRAHAM E. P., NEWTON G. G., HALE C. W., MILLER G. A. Incorporation of acetate into cephalosporin C. Biochem J. 1962 Jul;84:157–166. doi: 10.1042/bj0840157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROWN P. W., SHARP M., ABRAHAM E. P. alpha-Oxoglutarate as a precursor of the D-alpha-aminoadipic acid residue in cephalosporin C. Biochem J. 1963 Feb;86:280–284. doi: 10.1042/bj0860280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROWN P. W., SMITH B., ABRAHAM E. P. Biosynthesis of cephalosporin C from amino acids. Biochem J. 1963 Feb;86:284–291. doi: 10.1042/bj0860284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLNER D., MEISTER A. Studies on the mechanism of action of L-amino acid oxidase. J Biol Chem. 1961 Aug;236:2357–2364. [PubMed] [Google Scholar]
- Warren S. C., Newton G. G., Abraham E. P. The role of valine in the biosynthesis of penicillin N and cephalosporin C by a Cephalosporium sp. Biochem J. 1967 Jun;103(3):902–912. doi: 10.1042/bj1030902. [DOI] [PMC free article] [PubMed] [Google Scholar]


