Abstract
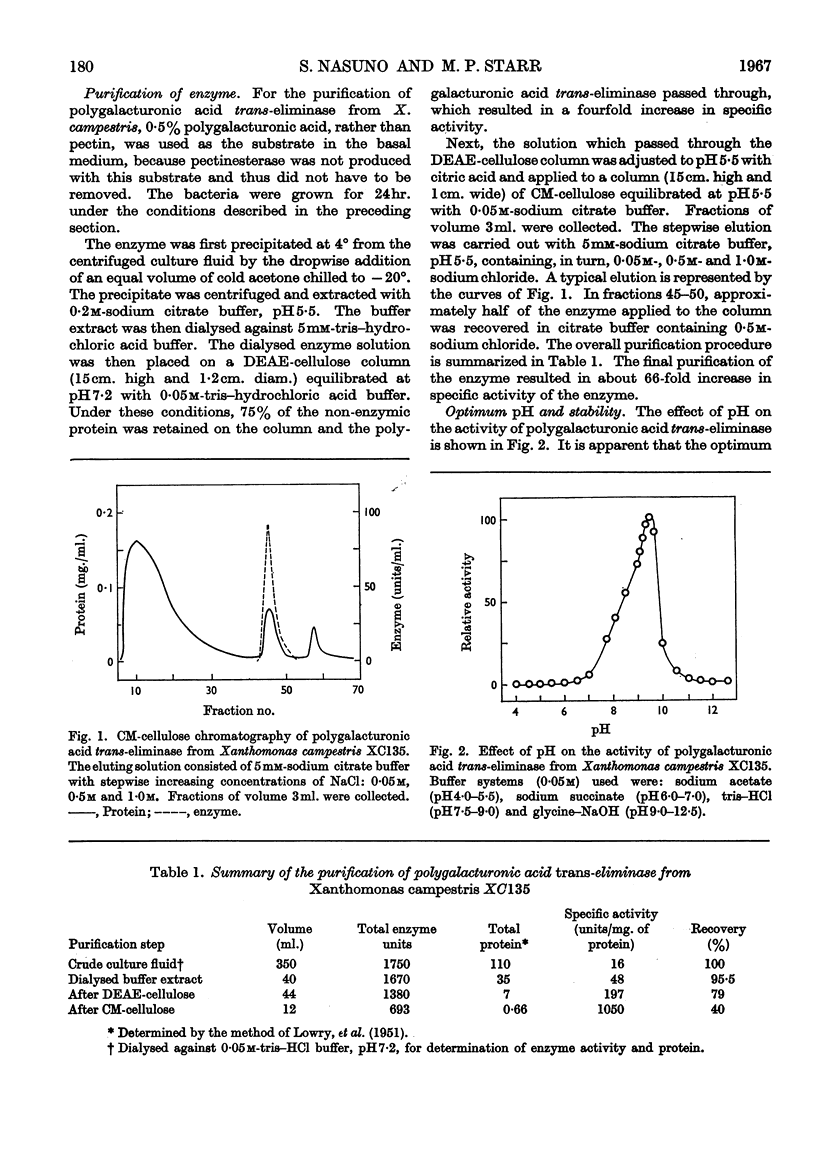
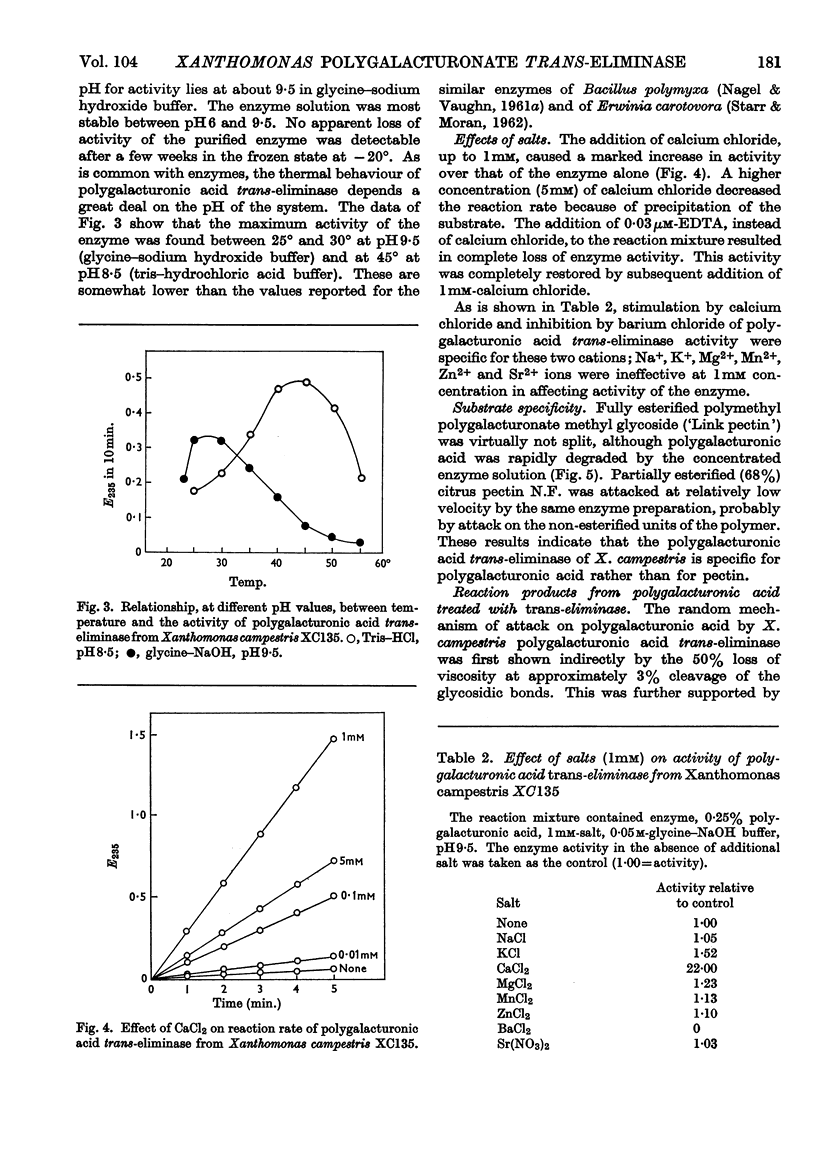
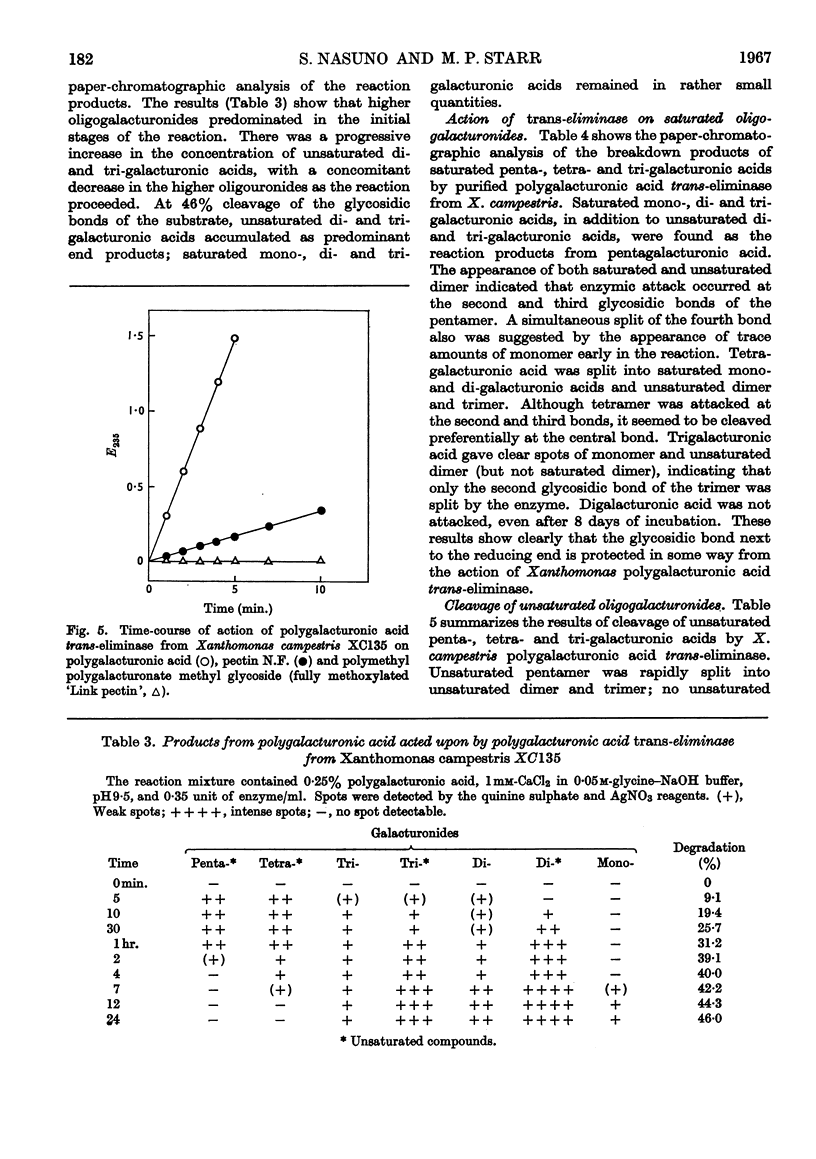
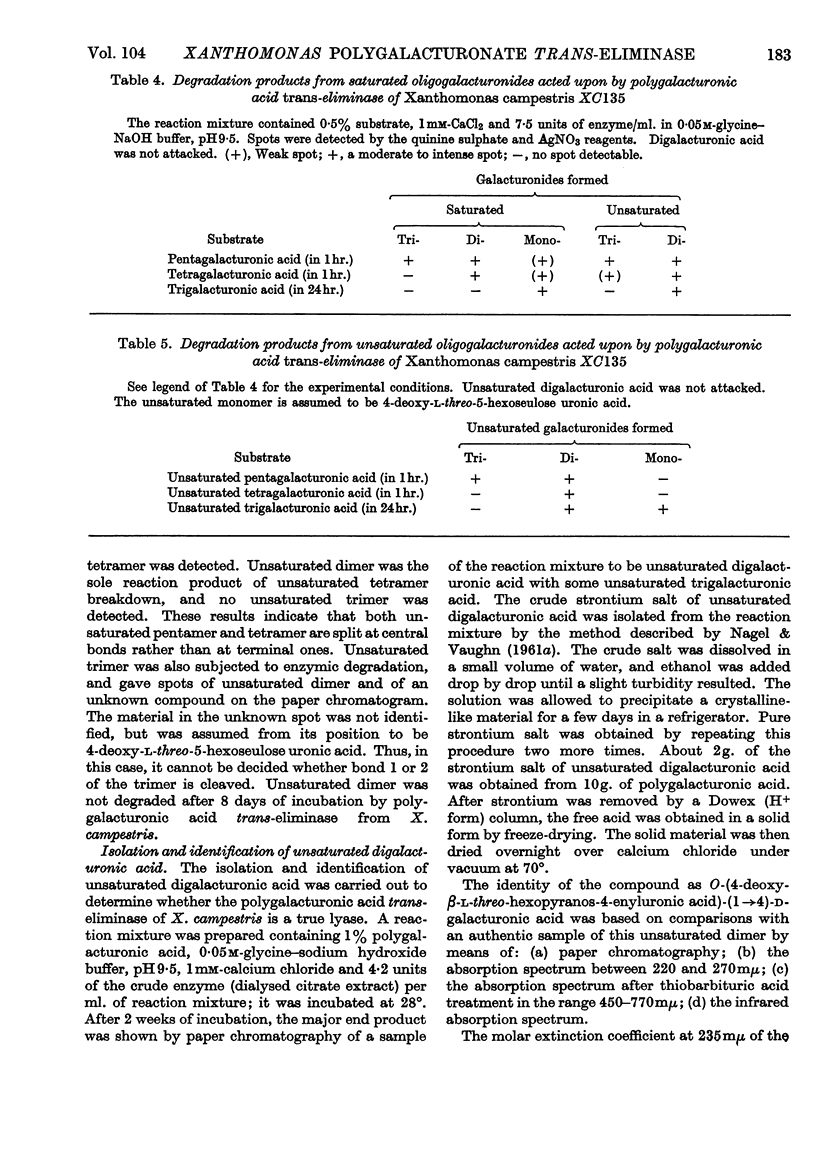
Polygalacturonic acid trans-eliminase from the culture fluid of Xanthomonas campestris was purified 66-fold by acetone precipitation, citrate extraction and chromatography on diethylaminoethyl- and carboxymethyl-cellulose. The optimum pH is 9·5 in glycine–sodium hydroxide buffer. Up to 1mm-calcium chloride brings about a remarkable stimulation of the enzyme activity and, at this concentration, no other cations promote or inhibit enzyme action except Ba2+ ions, which cause complete inhibition. The enzyme degrades polygalacturonic acid in a random manner; it does not act upon polygalacturonate methyl glycoside, although it can cleave partially (68%) esterified pectin. The end products from polygalacturonic acid at 46% breakdown are unsaturated di- and tri-galacturonic acids, in addition to saturated mono-, di- and tri-galacturonic acids. Pentagalacturonic acid is split preferentially into saturated dimer plus unsaturated trimer, or into saturated trimer plus unsaturated dimer; at a lower rate, it is also split into monomer and unsaturated tetramer. Unsaturated pentamer is split into unsaturated dimer plus unsaturated trimer. Tetragalacturonic acid is split some-what preferentially at the central bond to form dimer and unsaturated dimer, but it is also split into monomer and unsaturated trimer. Unsaturated tetramer is split only at the central bond to yield only unsaturated dimer. Trigalacturonic acid is split into monomer and unsaturated dimer. Unsaturated trimer is cleaved into saturated dimer and probably 4-deoxy-l-5-threo-hexoseulose uronic acid, which has not yet been directly identified. Neither saturated nor unsaturated digalacturonic acid is attacked. The unsaturated digalacturonic acid was isolated and proved to be O-(4-deoxy-β-l-5-threo-hexopyranos-4-enyluronic acid)-(1→4)-d-galacturonic acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DEMAIN A. L., PHAFF H. J. The preparation of tetragalacturonic acid. Arch Biochem Biophys. 1954 Jul;51(1):114–121. doi: 10.1016/0003-9861(54)90459-2. [DOI] [PubMed] [Google Scholar]
- EDSTROM R. D., PHAFF H. J. ELIMINATIVE CLEAVAGE OF PECTIN AND OF OLIGOGALACTURONIDE METHYL ESTERS BY PECTIN TRANS-ELIMINASE. J Biol Chem. 1964 Aug;239:2409–2415. [PubMed] [Google Scholar]
- Fuchs A. The trans-eliminative breakdown of Na-polygalacturonate by Pseudomonas fluorescens. Antonie Van Leeuwenhoek. 1965;31(3):323–340. doi: 10.1007/BF02045912. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUH B. S., PHAFF H. J. End products and mechanism of hydrolysis of pectin and pectic acid by yeast polygalacturonase (YPG). Arch Biochem Biophys. 1954 Jul;51(1):102–113. doi: 10.1016/0003-9861(54)90458-0. [DOI] [PubMed] [Google Scholar]
- LUH B. S., PHAFF H. J. Properties of yeast polygalacturonase. Arch Biochem Biophys. 1954 Jan;48(1):23–37. doi: 10.1016/0003-9861(54)90301-x. [DOI] [PubMed] [Google Scholar]
- MACMILLAN J. D., VAUGHN R. H. PURIFICATION AND PROPERTIES OF A POLYGALACTURONIC ACID-TRANS-ELIMINASE PRODUCED BY CLOSTRIDIUM MULTIFERMENTANS. Biochemistry. 1964 Apr;3:564–572. doi: 10.1021/bi00892a016. [DOI] [PubMed] [Google Scholar]
- NAGEL C. W., VAUGHN R. H. Comparison of growth and pectolytic enzyme production by Bacillus polymyxa. J Bacteriol. 1962 Jan;83:1–5. doi: 10.1128/jb.83.1.1-5.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGEL C. W., VAUGHN R. H. The characteristics of a polygalacturonase produced by Bacillus polymyxa. Arch Biochem Biophys. 1961 May;93:344–352. doi: 10.1016/0003-9861(61)90277-6. [DOI] [PubMed] [Google Scholar]
- NAGEL C. W., VAUGHN R. H. The degradation of oligogalacturonides by the polygalacturonase of Bacillus polymyxa. Arch Biochem Biophys. 1961 Aug;94:328–332. doi: 10.1016/0003-9861(61)90047-9. [DOI] [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonase of Erwinia carotovora. J Biol Chem. 1966 Nov 25;241(22):5298–5306. [PubMed] [Google Scholar]
- SMITH W. K. A survey of the production of pectic enzymes by plant pathogenic and other bacteria. J Gen Microbiol. 1958 Feb;18(1):33–41. doi: 10.1099/00221287-18-1-33. [DOI] [PubMed] [Google Scholar]
- SMITH W. K. Chromatographic examination of the products of digestion of pectic materials by culture solutions of plant pathogenic and other bacteria. J Gen Microbiol. 1958 Feb;18(1):42–47. doi: 10.1099/00221287-18-1-42. [DOI] [PubMed] [Google Scholar]
- STARR M. P., MORAN F. Eliminative split of pectic substances by phytopathogenic soft-rot bacteria. Science. 1962 Mar 16;135(3507):920–921. doi: 10.1126/science.135.3507.920. [DOI] [PubMed] [Google Scholar]


