Abstract
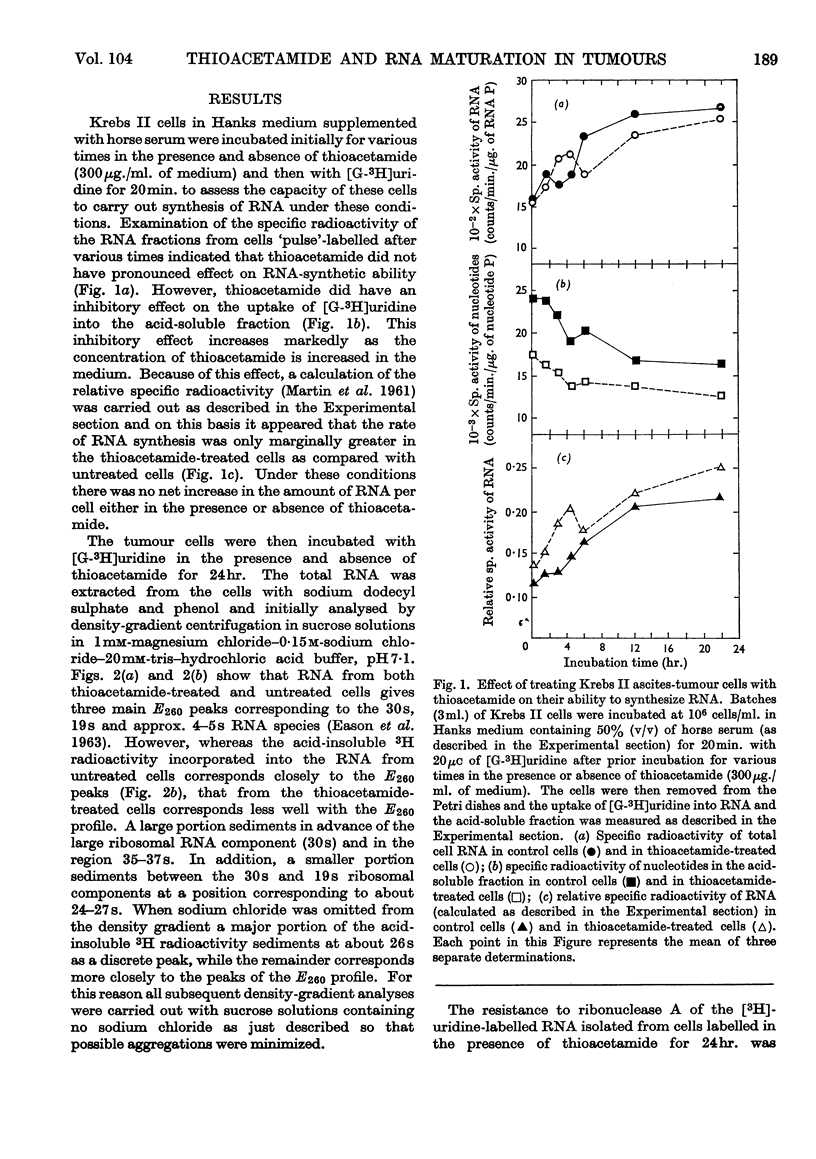
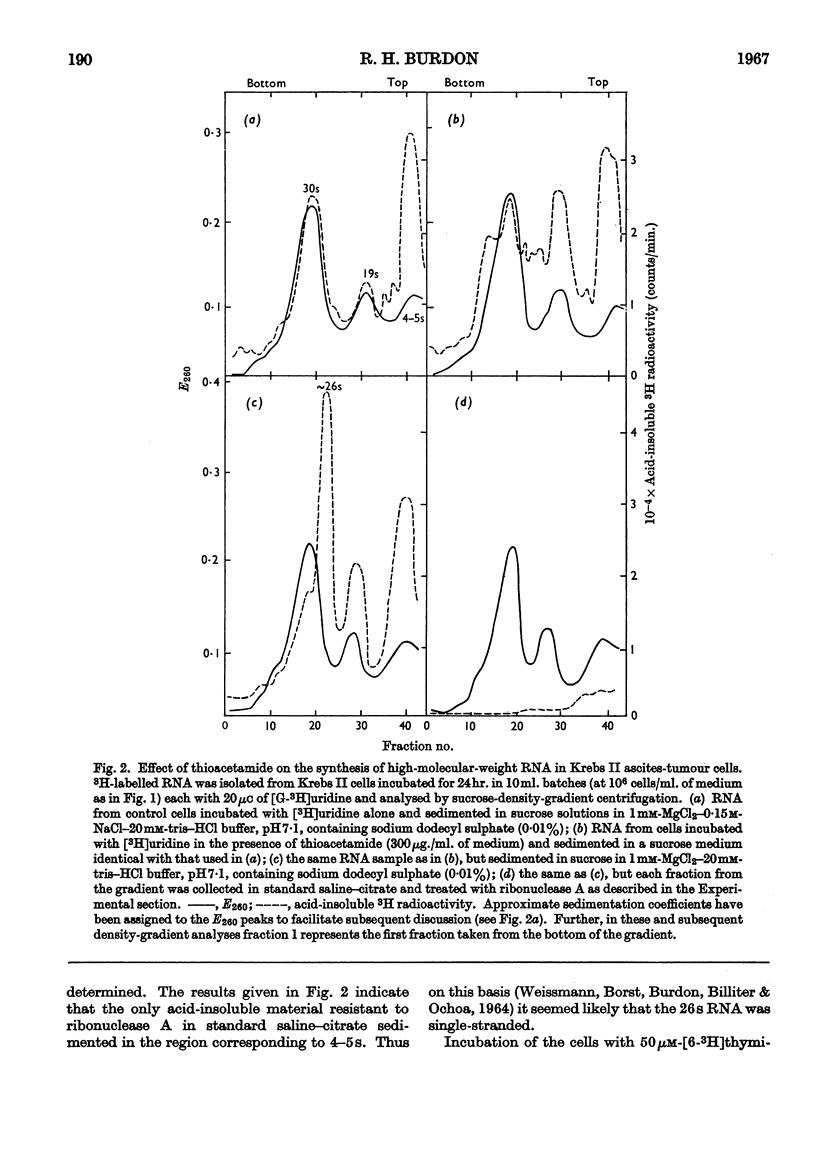
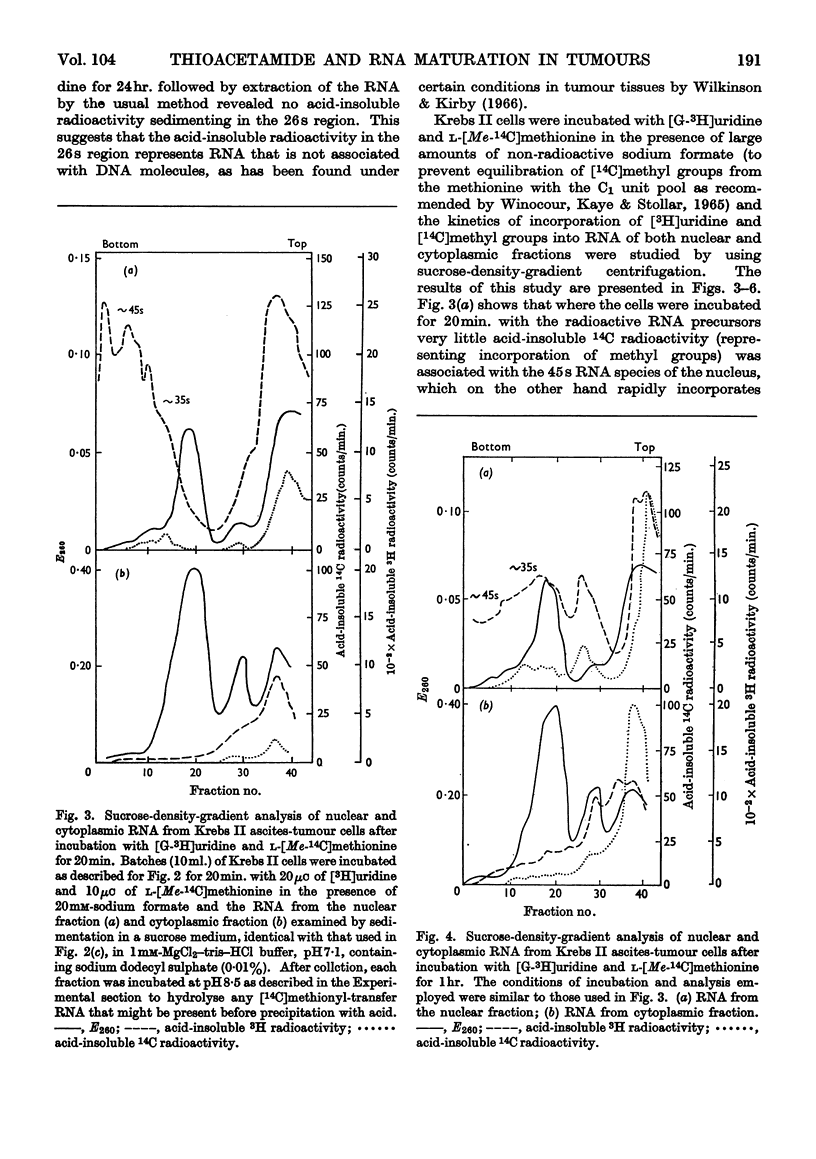
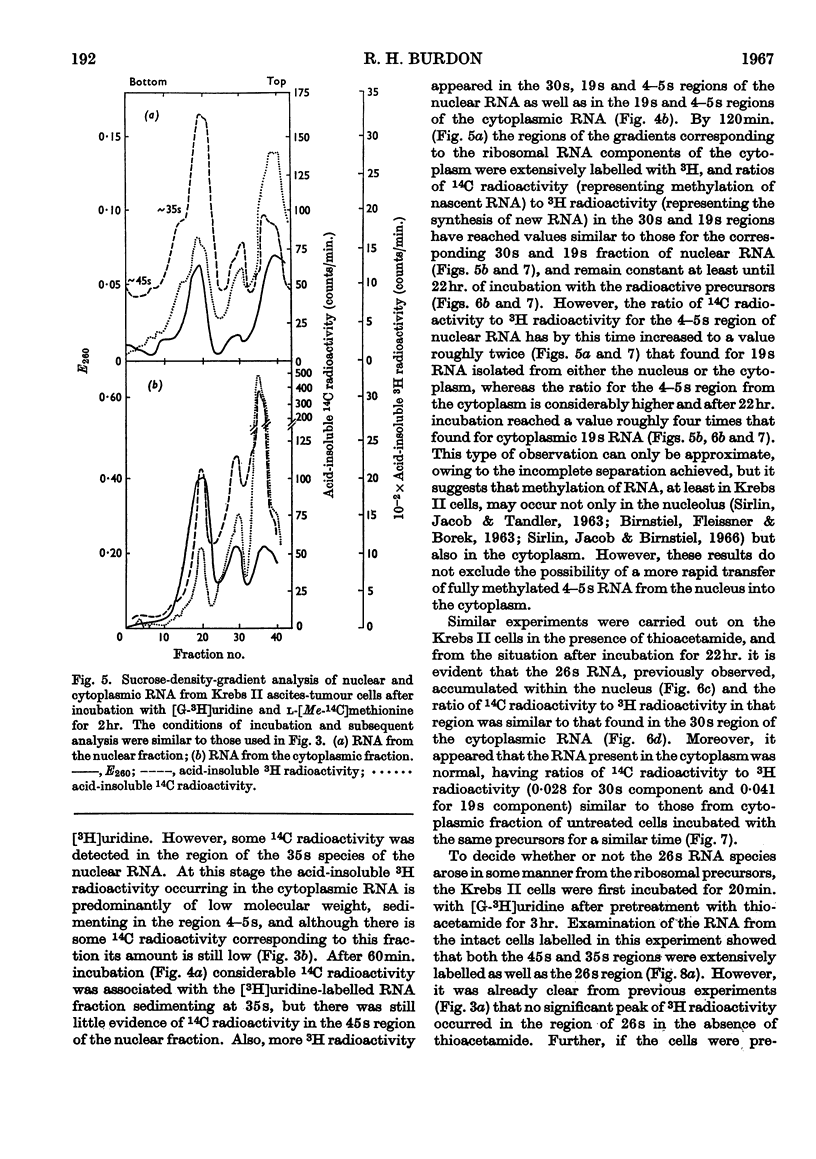
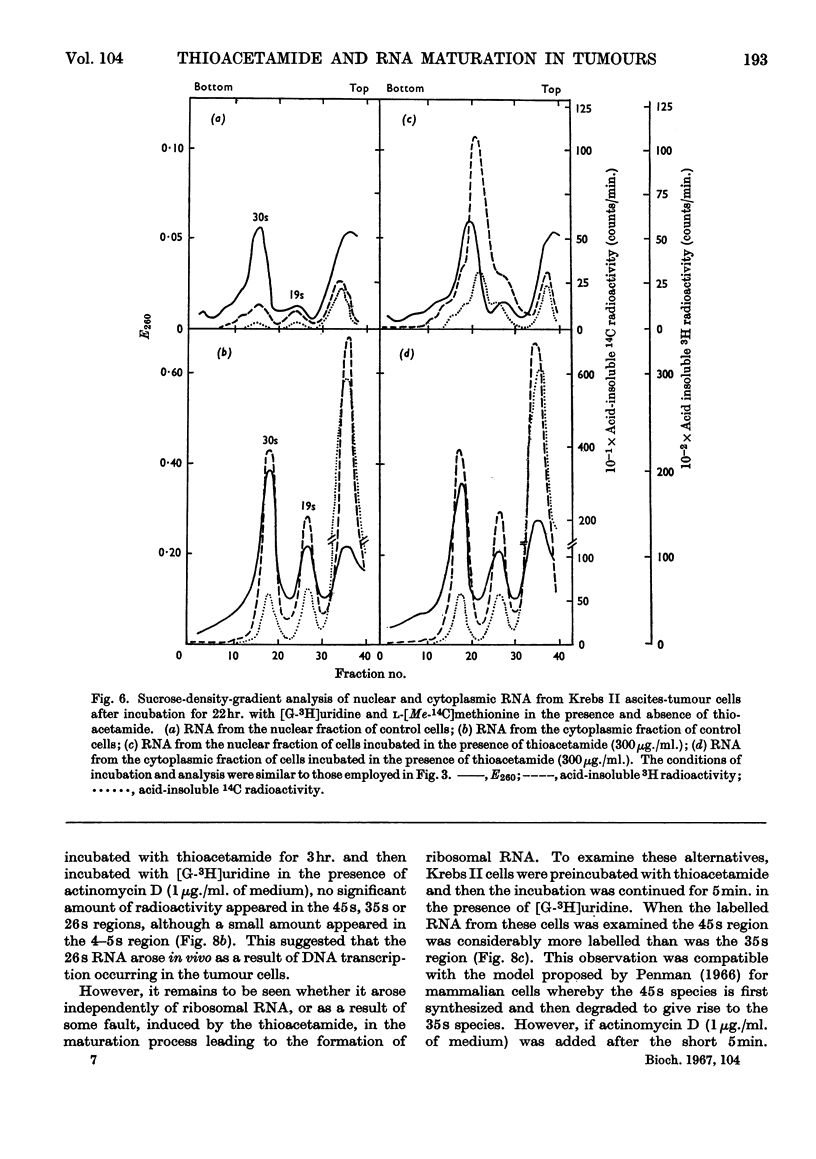
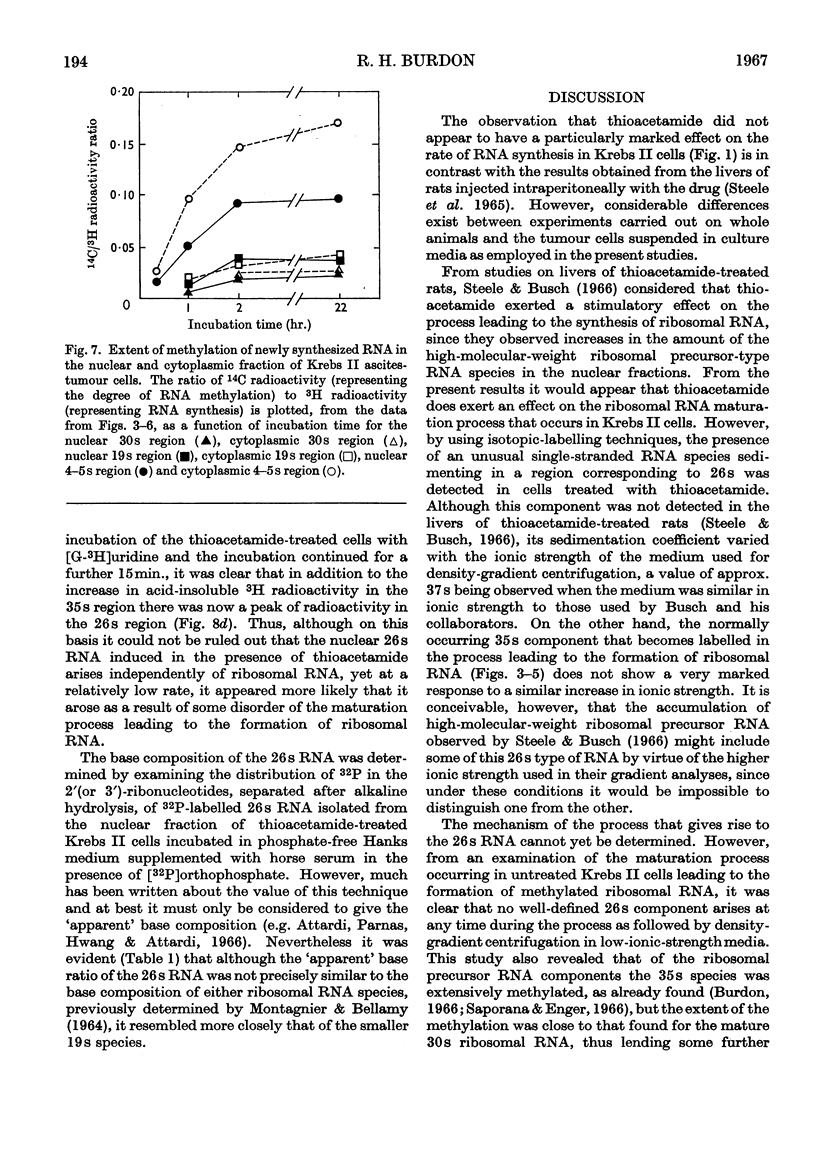
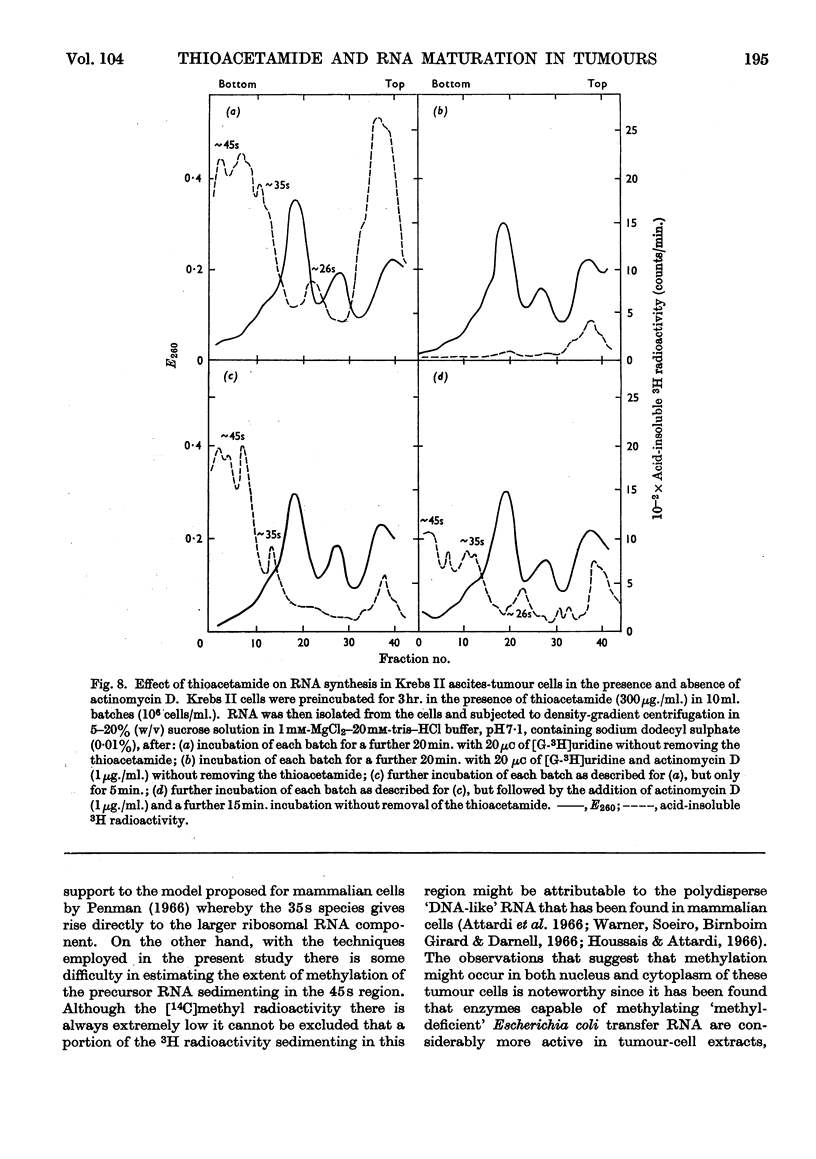
1. Although thioacetamide treatment of Krebs II ascites-tumour cells did not markedly affect the rate of RNA synthesis in vivo, it caused the formation of an unusual single-stranded RNA component sedimenting at approx. 26s. 2. The maturation process leading to the formation of methylated RNA was examined by following the kinetics of incorporation into RNA of radioactivity from [G-3H]uridine and l-[Me-14C]methionine. In treated and untreated tumour cells extensive methylation was observed, not only of the ribosomal RNA species, but also of their precursors, especially the precursor species sedimenting at 35s. 3. Evidence is also presented to suggest that methylation of low-molecular-weight RNA species occurs both in the nucleus and in the cytoplasm of these tumour cells. 4. Thioacetamide did not appear to have an effect on RNA methylation in vivo, and in thioacetamide-treated cells the 26s RNA accumulated within the nucleus, where it was methylated. 5. It is postulated that the 26s RNA is most likely to arise as a result of a fault in the scission process that gives rise to the ribosomal RNA components from their high-molecular-weight precursors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Parnas H., Hwang M. I., Attardi B. Giant-size rapidly labeled nuclear ribonucleic acid and cytoplasmic messenger ribonucleic acid in immature duck erythrocytes. J Mol Biol. 1966 Sep;20(1):145–182. doi: 10.1016/0022-2836(66)90123-9. [DOI] [PubMed] [Google Scholar]
- BIRNSTIEL M. L., FLEISSNER E., BOREK E. NUCLEOLUS: A CENTER OF RNA METHYLATION. Science. 1963 Dec 20;142(3599):1577–1580. doi: 10.1126/science.142.3599.1577. [DOI] [PubMed] [Google Scholar]
- BROWN D. D., LITTNA E. RNA SYNTHESIS DURING THE DEVELOPMENT OF XENOPUS LAEVIS, THE SOUTH AFRICAN CLAWED TOAD. J Mol Biol. 1964 May;8:669–687. doi: 10.1016/s0022-2836(64)80116-9. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Roberts R. B. High-Resolution Density Gradient Sedimentation Analysis. Science. 1960 Jan 1;131(3392):32–33. doi: 10.1126/science.131.3392.32. [DOI] [PubMed] [Google Scholar]
- Burdon R. H. Methylation of nucleic acids in Krebs II ascites tumour cells. Nature. 1966 May 21;210(5038):797–799. doi: 10.1038/210797a0. [DOI] [PubMed] [Google Scholar]
- EASON R., CLINE M. J., SMELLIE R. M. RIBONUCLEIC ACIDS IN VIRUS-INFECTED AND UNINFECTED KREBS II ASCITES TUMOR CELLS. J Biol Chem. 1963 Dec;238:3978–3984. [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]
- Houssais J. F., Attardi G. High molecular weight nonribosomal-type nuclear RNA and cytoplasmic messenger RNA in HeLa cells. Proc Natl Acad Sci U S A. 1966 Aug;56(2):616–623. doi: 10.1073/pnas.56.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN E. M., MALEC J., SVED S., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. 1. Encephalomyocarditis virus in Krebs II mouse-ascites-tumour cells. Biochem J. 1961 Sep;80:585–597. doi: 10.1042/bj0800585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MCNAMARA P. RNA SYNTHESIS IN KREBS II ASCITES TUMOUR CELLS. Life Sci. 1964 Dec;3:1437–1447. doi: 10.1016/0024-3205(64)90086-4. [DOI] [PubMed] [Google Scholar]
- Muramatsu M., Hodnett J. L., Steele W. J., Busch H. Synthesis of 28-S RNA in the nucleolus. Biochim Biophys Acta. 1966 Jul 20;123(1):116–125. doi: 10.1016/0005-2787(66)90164-x. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- RAKE A. V., GRAHAM A. F. KINETICS OF INCORPORATION OF URIDINE-C14 INTO L CELL RNA. Biophys J. 1964 Jul;4:267–284. doi: 10.1016/s0006-3495(64)86782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER K., LATHAM H., DARNELL J. E. Demonstration of an unstable RNA and of a precursor to ribosomal RNA in HeLa cells. Proc Natl Acad Sci U S A. 1963 Feb 15;49:240–248. doi: 10.1073/pnas.49.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRLIN J. L., JACOB J., TANDLER C. J. TRANSFER OF THE METHYL GROUP OF METHIONINE TO NUCLEOLAR RIBONUCLEIC ACID. Biochem J. 1963 Dec;89:447–452. doi: 10.1042/bj0890447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEELE W. J., OKAMURA N., BUSCH H. EFFECTS OF THIOACETAMIDE ON THE COMPOSITION AND BIOSYNTHESIS OF NUCLEOLAR AND NUCLEAR RIBONUCLEIC ACID IN RAT LIVER. J Biol Chem. 1965 Apr;240:1742–1749. [PubMed] [Google Scholar]
- Saponara A. G., Enger M. D. Incorporation of [3-H]uridine and [M3-14-C]methionine into Chinese-hamster cell ribonucleic acid. Biochim Biophys Acta. 1966 Jun 22;119(3):492–500. [PubMed] [Google Scholar]
- Srinivasan P. R., Borek E. Enzymatic alteration of macromolecular structure. Prog Nucleic Acid Res Mol Biol. 1966;5:157–189. doi: 10.1016/s0079-6603(08)60234-2. [DOI] [PubMed] [Google Scholar]
- Steele W. J., Busch H. Increased content of high molecular weight RNA fractions in nuclei and nucleoli of livers of thioacetamide-treated rats. Biochim Biophys Acta. 1966 Jun 22;119(3):501–509. doi: 10.1016/0005-2787(66)90126-2. [DOI] [PubMed] [Google Scholar]
- WEISSMANN C., BORST P., BURDON R. H., BILLETER M. A., OCHOA S. REPLICATION OF VIRAL RNA, III. DOUBLE-STRANDED REPLICATIVE FORM OF MSW PHAGE RNA. Proc Natl Acad Sci U S A. 1964 Apr;51:682–690. doi: 10.1073/pnas.51.4.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson B. R., Kirby K. S. Studies in rapidly labelled ribonucleic acid in cell fractions and in tumour tissues. Biochem J. 1966 Jun;99(3):780–785. doi: 10.1042/bj0990780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Kaye A. M., Stollar V. Synthesis and transmethylation of DNA in polyoma-infected cultures. Virology. 1965 Oct;27(2):156–169. doi: 10.1016/0042-6822(65)90155-8. [DOI] [PubMed] [Google Scholar]


