Abstract
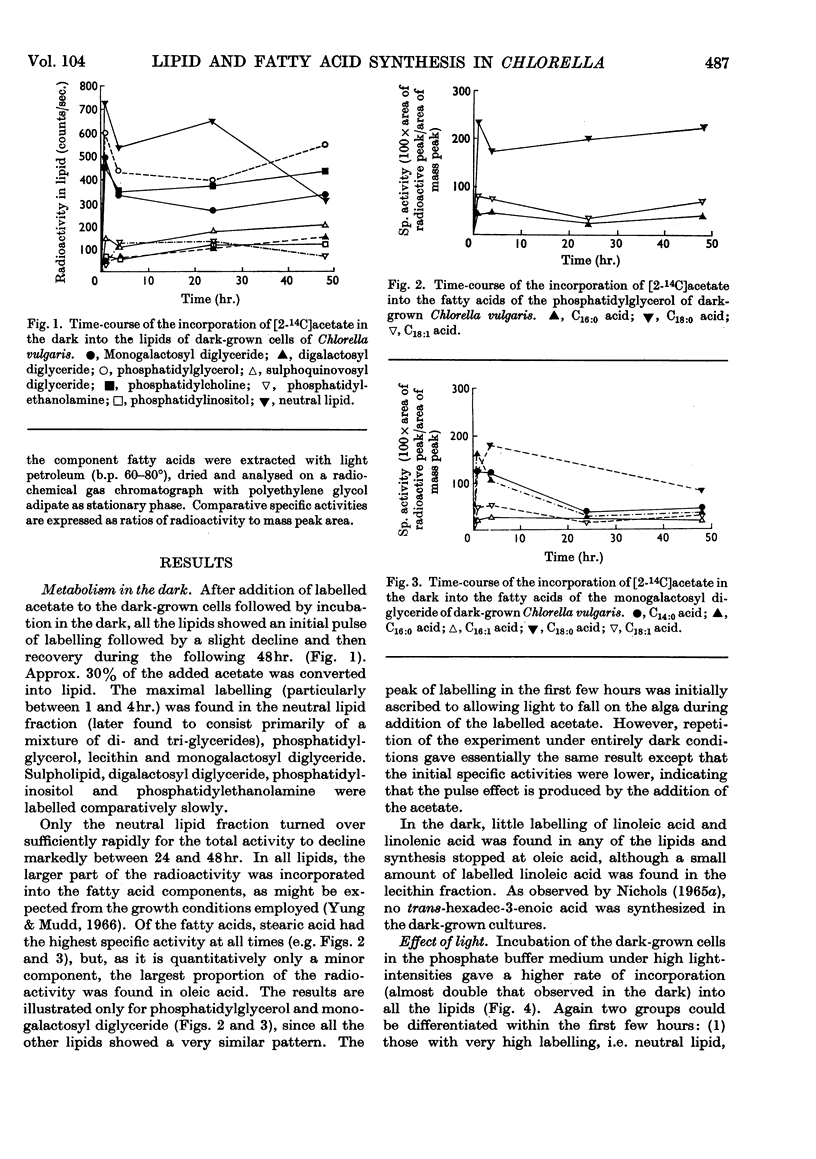
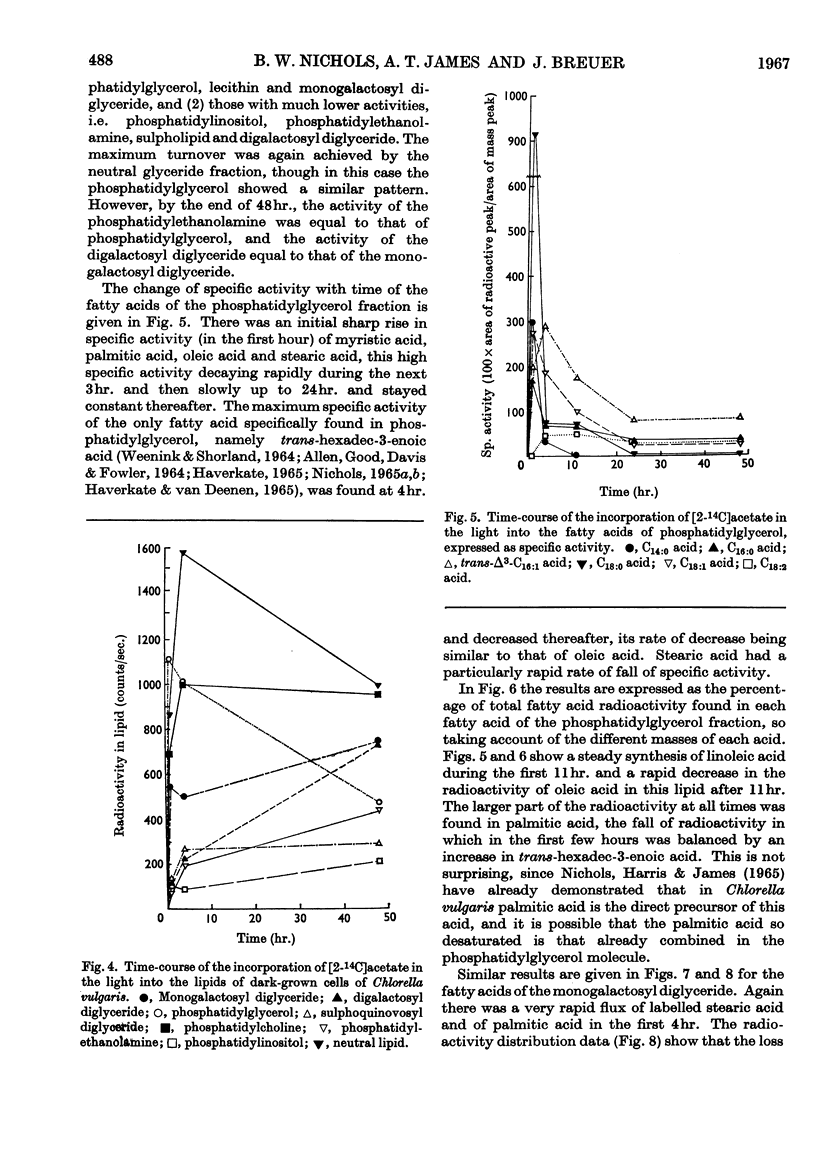
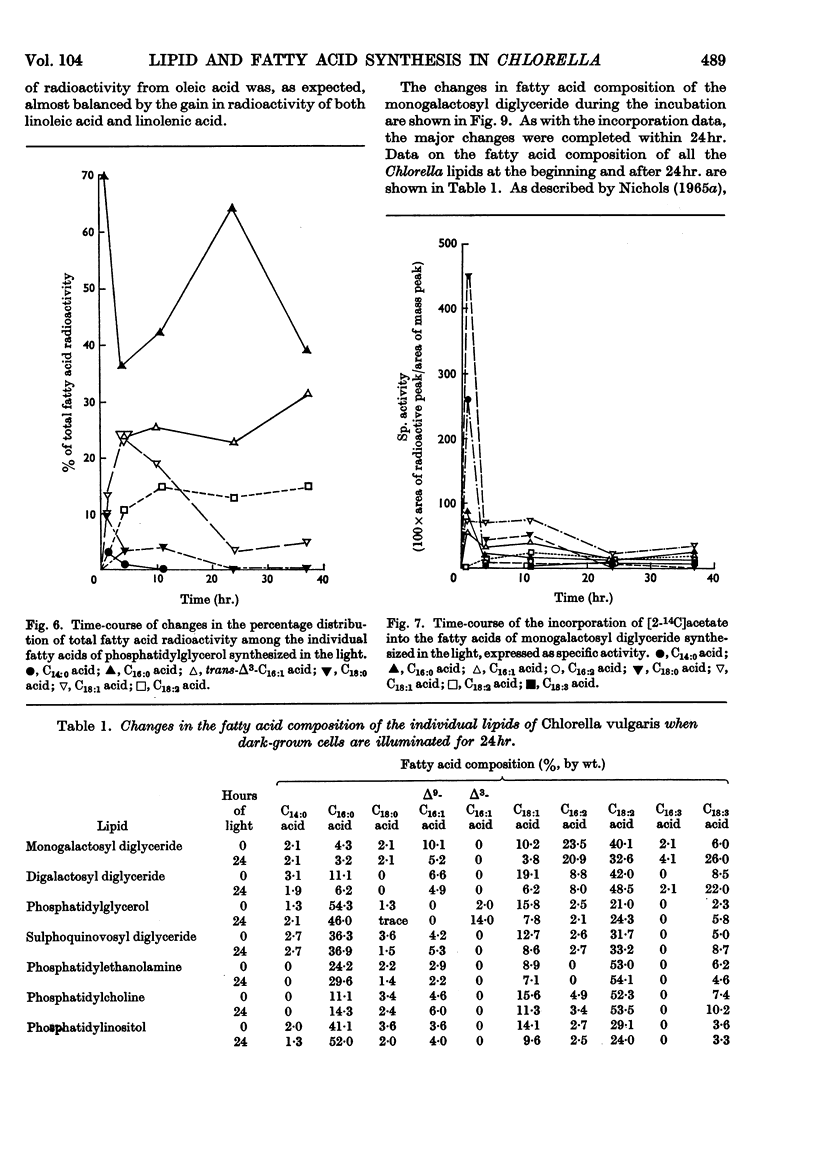
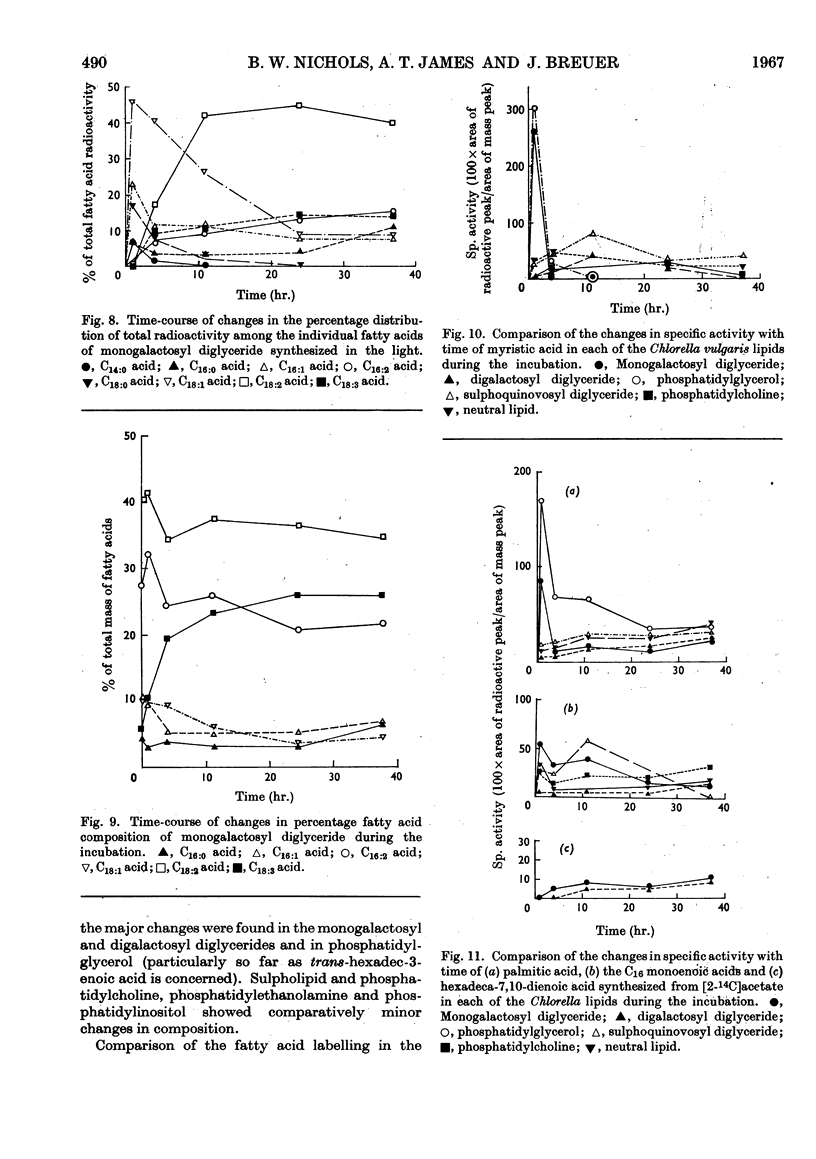
1. Fatty acid synthesis from [2-14C]acetate by Chlorella vulgaris cells grown and incubated in the dark is limited almost entirely to the production of saturated and monoenoic acids. 2. In light-incubated cells, both saturated and polyunsaturated fatty acids are rapidly synthesized. 3. Two groups of lipids can be distinguished in both dark- and light-incubated cells. The first group, consisting of phosphatidyl-glycerol, monogalactosyl diglyceride, lecithin and neutral glyceride, has a very high turnover rate for certain fatty acids. The second group, consisting of digalactosyl diglyceride, sulpholipid, phosphatidylethanolamine and phosphatidylinositol, has a slow turnover of fatty acids. 4. The lipids with rapid fatty acid turnover may be involved in the sequences of saturated and unsaturated fatty acid synthesis. A classification of lipids is made on the basis of their suggested functions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C. F., Good P., Davis H. F., Fowler S. D. Plant and chloroplast lipids. I. Separation and composition of major spinach lipids. Biochem Biophys Res Commun. 1964 Apr 22;15(5):424–430. doi: 10.1016/0006-291x(64)90479-6. [DOI] [PubMed] [Google Scholar]
- FERRARI R. A., BENSON A. A. The path of carbon in photosynthesis of the lipids. Arch Biochem Biophys. 1961 May;93:185–192. doi: 10.1016/0003-9861(61)90248-x. [DOI] [PubMed] [Google Scholar]
- Harris R. V., James A. T. Linoleic and alpha-linolenic acid biosynthesis in plant leaves and green alga. Biochim Biophys Acta. 1965 Dec 2;106(3):456–464. doi: 10.1016/0005-2760(65)90062-7. [DOI] [PubMed] [Google Scholar]
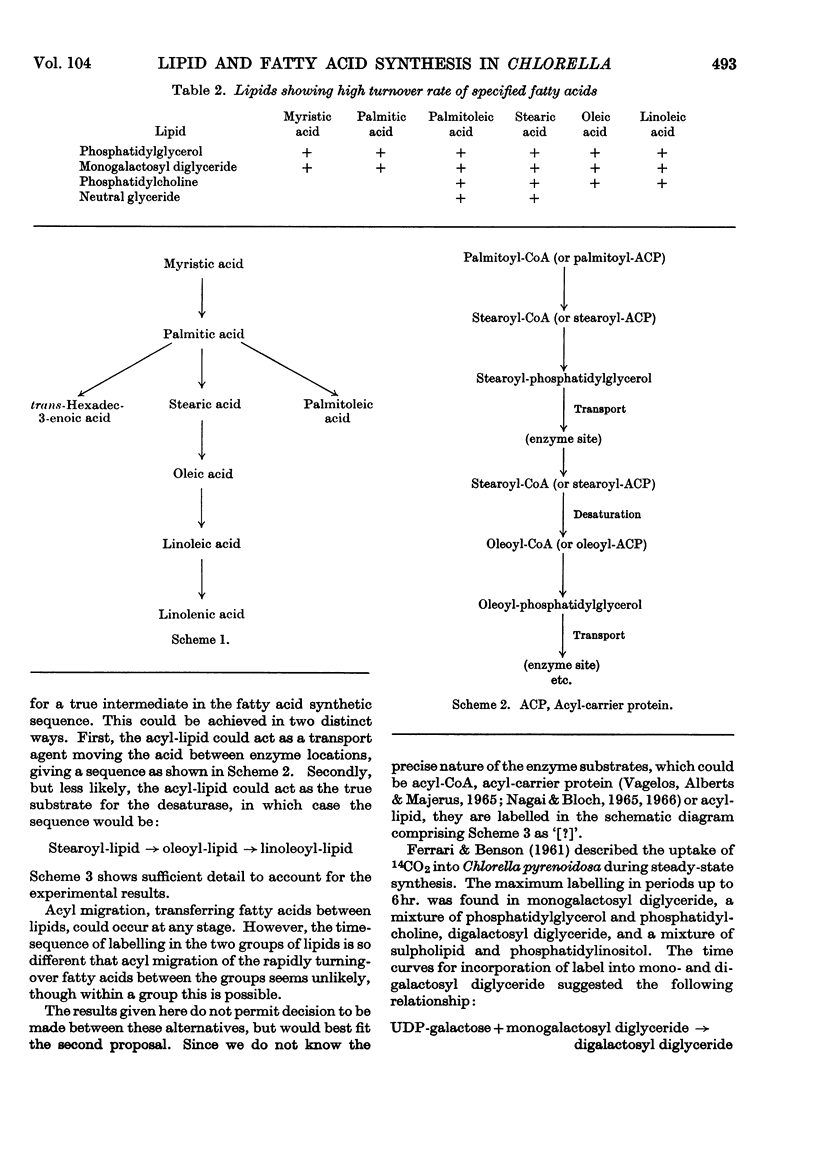
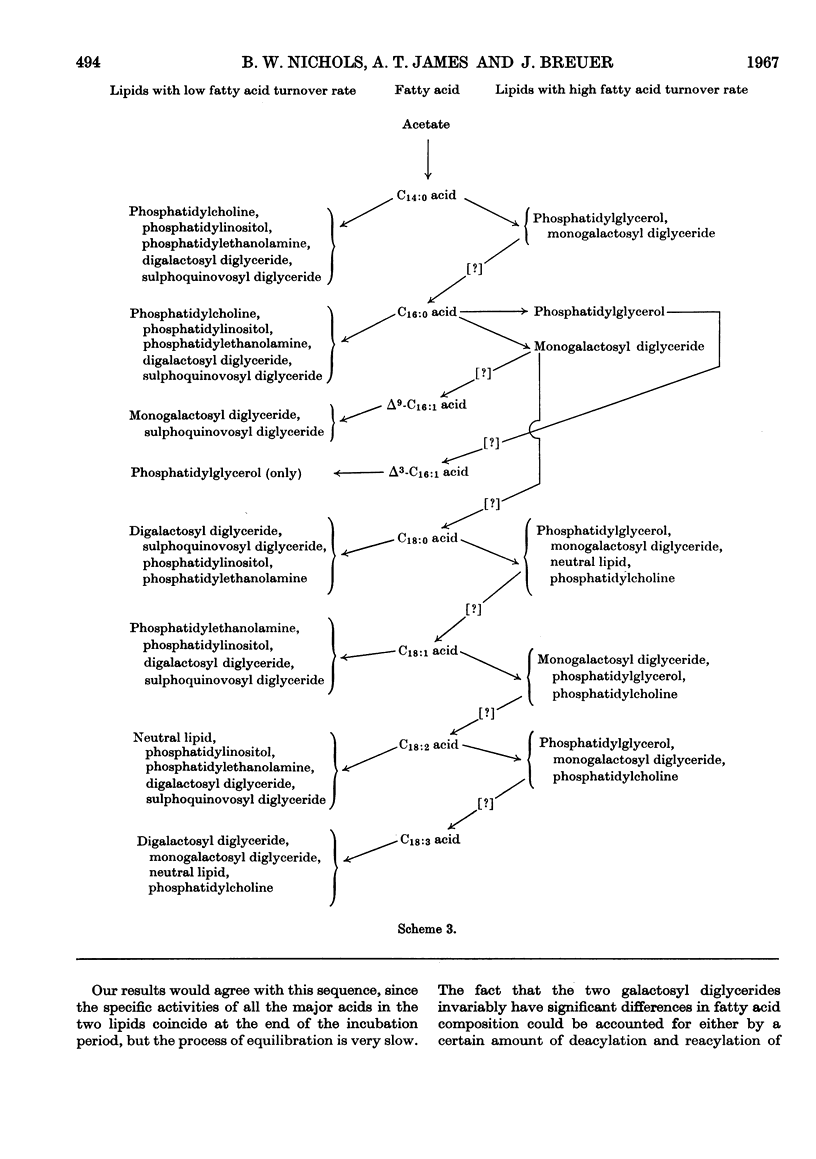
- Harris R. V., James A. T. The fatty acid metabolism of Chlorella vulgaris. Biochim Biophys Acta. 1965 Dec 2;106(3):465–473. doi: 10.1016/0005-2760(65)90063-9. [DOI] [PubMed] [Google Scholar]
- Miyachi S., Miyachi S. Sulfolipid metabolism in chlorella. Plant Physiol. 1966 Mar;41(3):479–486. doi: 10.1104/pp.41.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder E., van Deenen L. L. Metabolism of red-cell lipids. I. Incorporation in vitro of fatty acids into phospholipids from mature erythrocytes. Biochim Biophys Acta. 1965 Jul 7;106(1):106–117. doi: 10.1016/0005-2760(65)90099-8. [DOI] [PubMed] [Google Scholar]
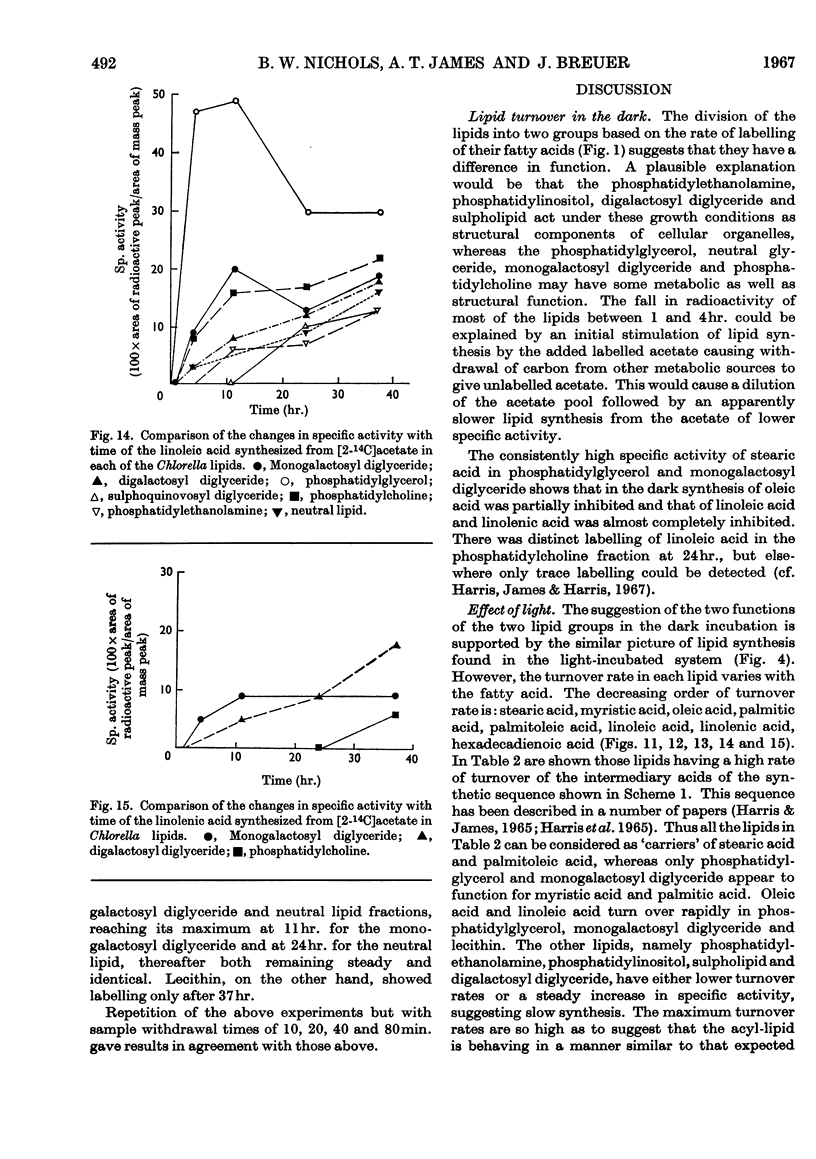
- Nagai J., Bloch K. Enzymatic desaturation of stearyl acyl carrier protein. J Biol Chem. 1966 Apr 25;241(8):1925–1927. [PubMed] [Google Scholar]
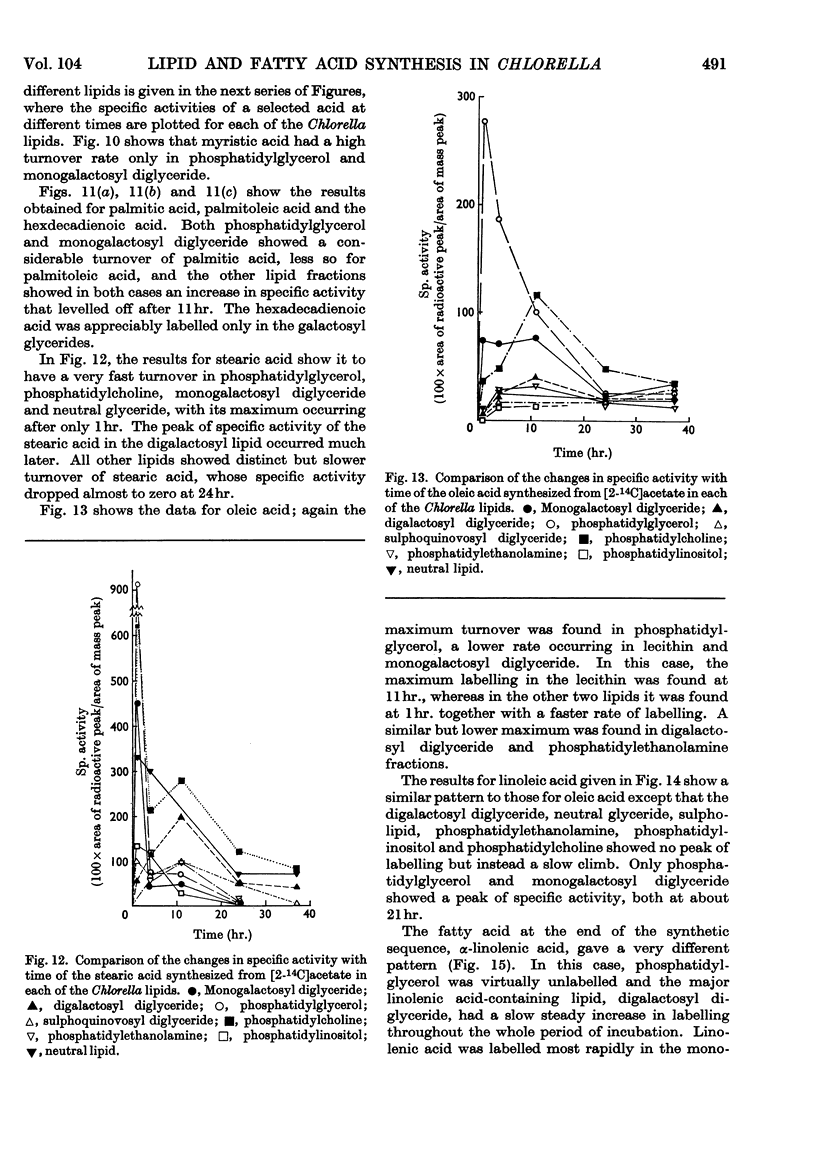
- Nichols B. W., Harris P., James A. T. The biosynthesis of trans-delta-3-hexadecenoic acid by chlorella vulgaris. Biochem Biophys Res Commun. 1965 Dec 9;21(5):473–479. doi: 10.1016/0006-291x(65)90407-9. [DOI] [PubMed] [Google Scholar]
- Nichols B. W. Light induced changes in the lipids of Chlorella vulgaris. Biochim Biophys Acta. 1965 Oct 4;106(2):274–279. doi: 10.1016/0005-2760(65)90035-4. [DOI] [PubMed] [Google Scholar]
- Sastry P. S., Kates M. Biosynthesis of lipids in plants. I. Incorporation of orthophosphate-32P and glycerophosphate-32P into phosphatides of Chlorella vulgaris during photosynthesis. Can J Biochem. 1965 Sep;43(9):1445–1453. doi: 10.1139/o65-162. [DOI] [PubMed] [Google Scholar]
- Vagelos P. R., Alberts A. W., Majerus P. W. The mechanism of fatty acid biosynthesis and the involvement of an acyl carrier protein. Ann N Y Acad Sci. 1965 Oct 8;131(1):177–188. doi: 10.1111/j.1749-6632.1965.tb34787.x. [DOI] [PubMed] [Google Scholar]
- WEENINK R. O., SHORLAND F. B. THE ISOLATION OF TRANS-3-HEXADECENOIC ACID FROM THE LIPIDS OF RED-CLOVER (TRIFOLIUM PRATENSE) LEAVES. Biochim Biophys Acta. 1964 Oct 2;84:613–614. doi: 10.1016/0926-6542(64)90133-7. [DOI] [PubMed] [Google Scholar]


