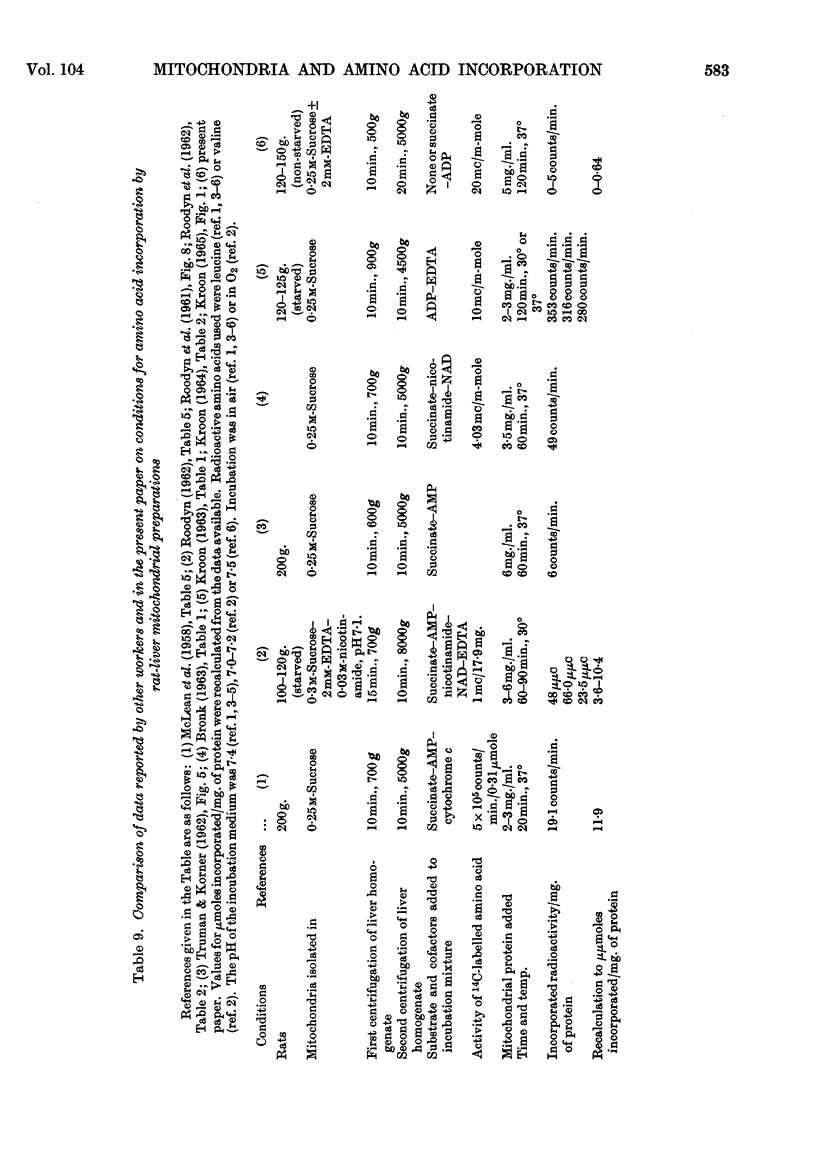
Abstract
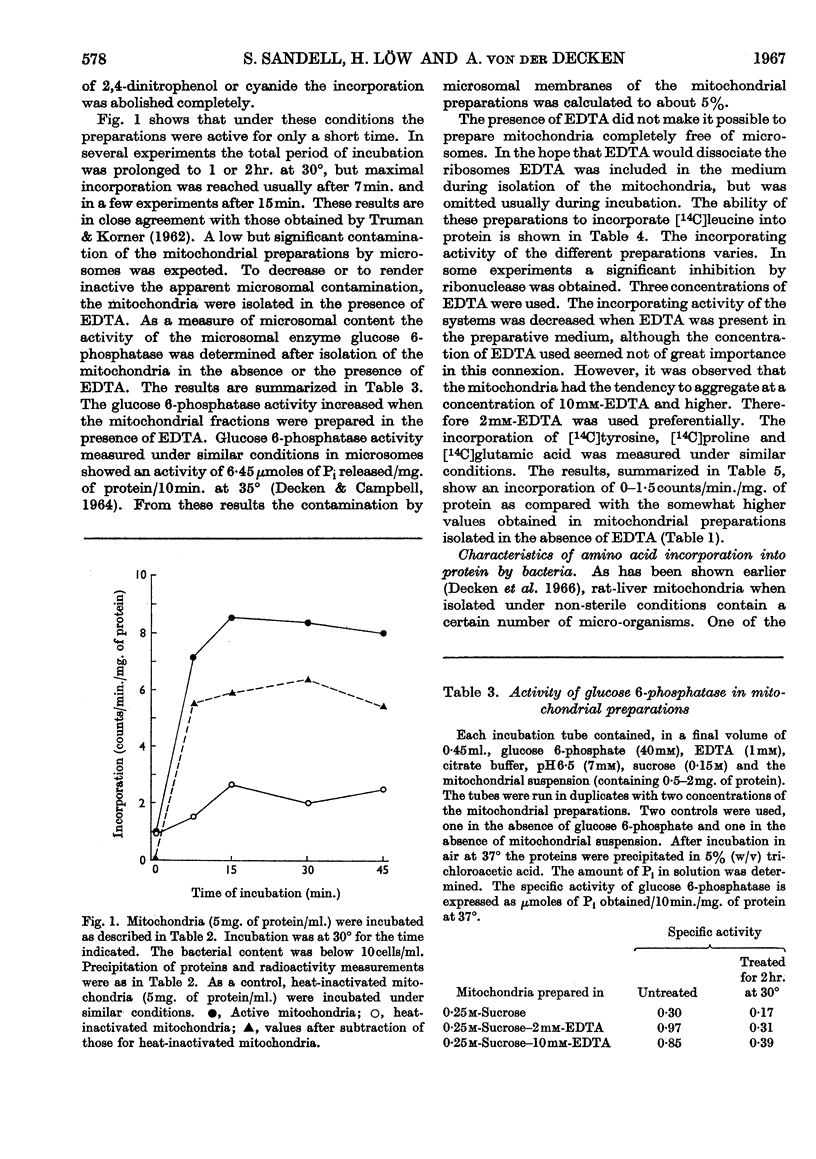
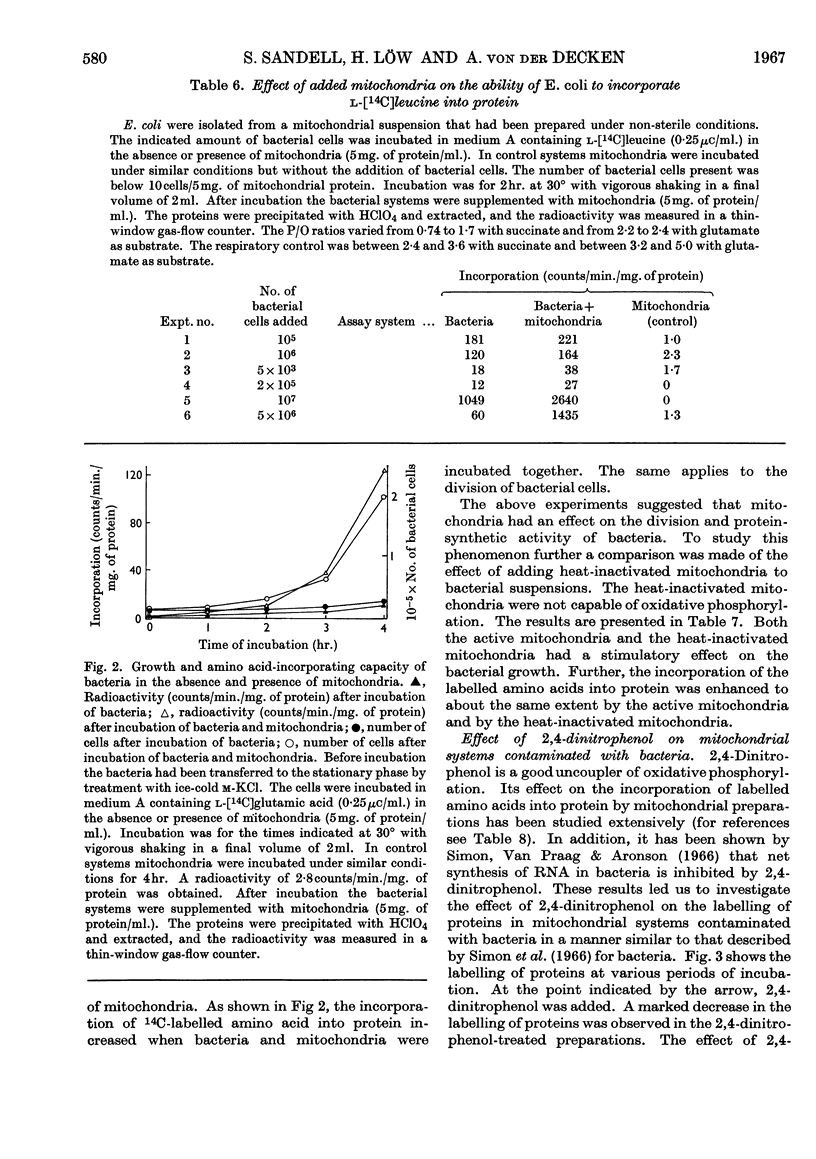
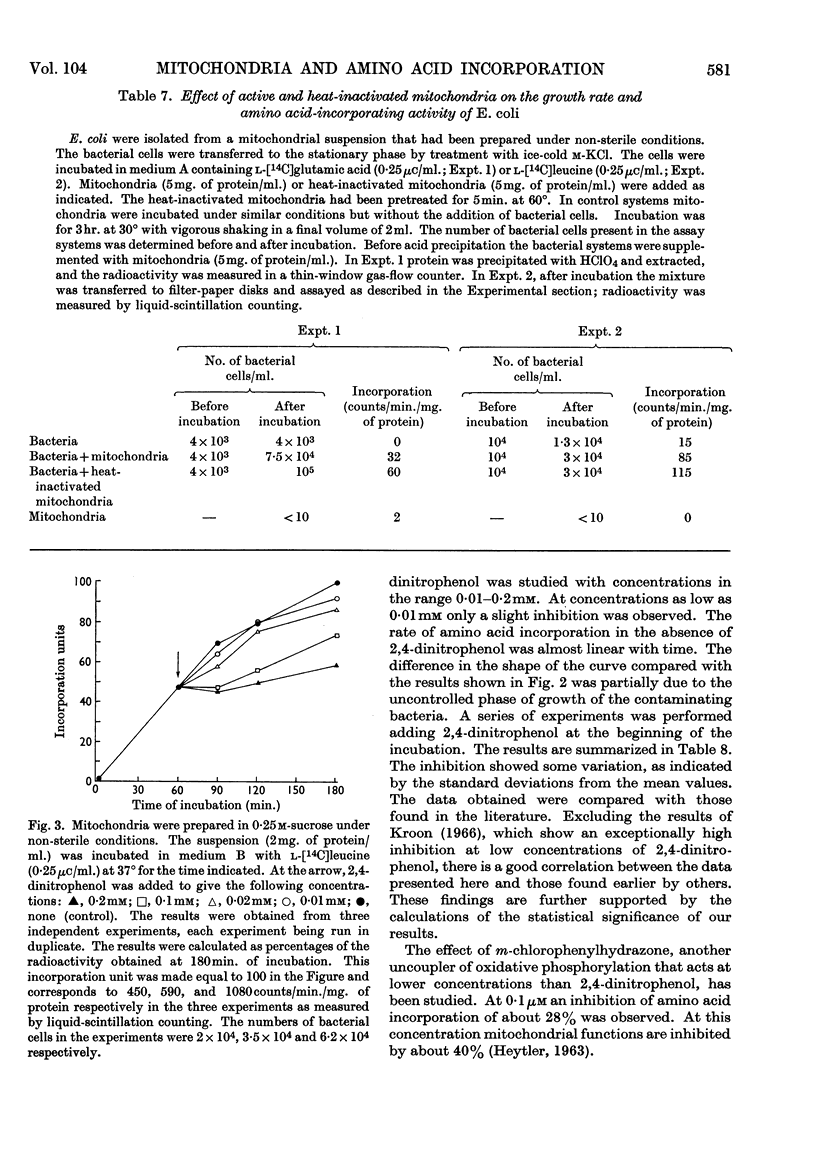
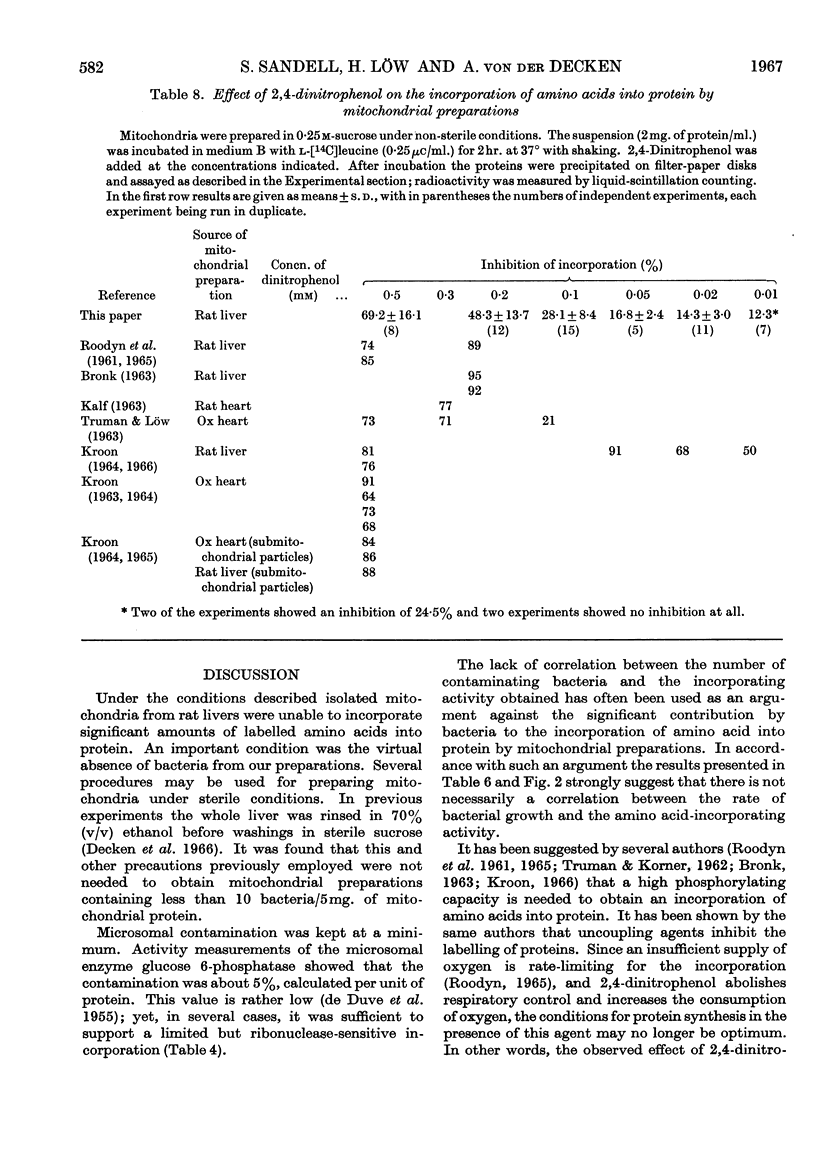
1. Mitochondria were isolated from rat liver in a way that kept bacterial contamination at a minimum. 2. The activity of oxidative phosphorylation was unchanged under these conditions, whereas the ability of the preparations to incorporate amino acids into protein was insignificant, though it could be enhanced somewhat by the presence of EDTA. This enhancement was sensitive to ribonuclease. 3. The active time of incorporation did not exceed 15min. at 30°. 4. Microsomal contamination, as measured by glucose 6-phosphatase activity, was about 5%. 5. The ability of isolated bacteria to incorporate amino acids into protein was greatly enhanced by the addition of mitochondria or heat-inactivated mitochondria. 6. A correlation was found between the growth rate of bacteria and the amino acid-incorporating activity. 7. Amino acid incorporation by combined mitochondrial–bacterial systems was inhibited by 2,4-dinitrophenol. 8. The results confirm and extend the earlier findings made in our Laboratory that isolated liver mitochondria, when free from contaminating bacteria and obtained from adult rats, are not able to catalyse the incorporation of amino acids into protein at a measurable rate. 9. The results are discussed with special emphasis on the validity of these findings.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRONK J. R. THE NATURE OF THE ENERGY REQUIREMENT FOR AMINO ACID INCORPORATION BY ISOLATED MITOCHONDRIA AND ITS SIGNIFICANCE FOR THYROID HORMONE ACTION. Proc Natl Acad Sci U S A. 1963 Sep;50:524–526. doi: 10.1073/pnas.50.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERNSTER L., LOW H. Reconstruction of oxidative phosphorylation in aged mitochondrial systems. Exp Cell Res. 1955;(Suppl 3):133–153. [PubMed] [Google Scholar]
- HEYTLER P. G. uncoupling of oxidative phosphorylation by carbonyl cyanide phenylhydrazones. I. Some characteristics of m-Cl-CCP action on mitochondria and chloroplasts. Biochemistry. 1963 Mar-Apr;2:357–361. doi: 10.1021/bi00902a031. [DOI] [PubMed] [Google Scholar]
- KALF G. F. The incorporation of leucine-1-C-14 into the protein of rat heart sarcosomes: an investigation of optimal conditions. Arch Biochem Biophys. 1963 May;101:350–359. doi: 10.1016/s0003-9861(63)80023-5. [DOI] [PubMed] [Google Scholar]
- KROON A. M. PROTEIN SYNTHESIS IN MITOCHONDRIA. II. A COMPARISON OF MITOCHONDRIA FROM LIVER AND HEART WITH SPECIAL REFERENCE TO THE ROLE OF OXIDATIVE PHOSPHORYLATION. Biochim Biophys Acta. 1964 Sep 11;91:145–154. [PubMed] [Google Scholar]
- KROON A. M. Protein synthesis in heart mitochondria. I. Amino acid incorporation into the protein of isolated beefheart mitochondria and fractions derived from them by sonic oscillation. Biochim Biophys Acta. 1963 Jul 30;72:391–402. doi: 10.1016/0006-3002(63)90258-0. [DOI] [PubMed] [Google Scholar]
- King E. J., Abul-Fadl M. A., Walker P. G. King-Armstrong Phosphatase Estimation by the Determination of Liberated Phosphate. J Clin Pathol. 1951 Feb;4(1):85–91. doi: 10.1136/jcp.4.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon A. M. Protein synthesis in mitochondria. 3. On the effects of inhibitors on the incorporation of amino acids into protein by intact mitochondria and digitonin fractions. Biochim Biophys Acta. 1965 Oct 11;108(2):275–284. doi: 10.1016/0005-2787(65)90012-2. [DOI] [PubMed] [Google Scholar]
- LAMBORG M. R., ZAMECNIK P. C. Amino acid incorporation into protein by extracts of E. coli. Biochim Biophys Acta. 1960 Aug 12;42:206–211. doi: 10.1016/0006-3002(60)90782-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McLEAN J. R., COHN G. L., BRANDT I. K., SIMPSON M. V. Incorporation of labeled amino acids into the protein of muscle and liver mitochondria. J Biol Chem. 1958 Sep;233(3):657–663. [PubMed] [Google Scholar]
- ROODYN D. B., FREEMAN K. B., TATA J. R. THE STIMULATION BY TREATMENT IN VIVO WITH TRI-IODOTHYRONINE OF AMINO ACID INCORPORATION INTO PROTEIN BY ISOLATED RAT-LIVER MITOCHONDRIA. Biochem J. 1965 Mar;94:628–641. doi: 10.1042/bj0940628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B. Protein synthesis in mitochondria. 3. The controlled disruption and subfractionation of mitochondria labelled in vitro with radioactive valine. Biochem J. 1962 Oct;85:177–189. doi: 10.1042/bj0850177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., REIS P. J., WORK T. S. Protein synthesis in mitochondria. Requirements for the incorporation of radioactive amino acids into mitochondrial protein. Biochem J. 1961 Jul;80:9–21. doi: 10.1042/bj0800009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., SUTTIE J. W., WORK T. S. Protein synthesis in mitochondria. 2. Rate of incorporation in vitro of radioactive amino acids into soluble proteins in the mitochondrial fraction, including catalase, malic dehydrogenase and cytochrome c. Biochem J. 1962 Apr;83:29–40. doi: 10.1042/bj0830029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodyn D. B. Further study of factors affecting amino acid incorporation into protein by isolated mitochondria. Biochem J. 1965 Dec;97(3):782–793. doi: 10.1042/bj0970782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTTIE J. W. The existence of two routes for incorporation of amino acids into protein of isolated rat-liver mitochondria. Biochem J. 1962 Aug;84:382–386. doi: 10.1042/bj0840382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. J., Van Praag D., Aronson F. L. The selective inhibition of ribosomal RNA synthesis in E. coli by 2,4-Dinitrophenol. Mol Pharmacol. 1966 Jan;2(1):43–49. [PubMed] [Google Scholar]
- TRUMAN D. E., KORNER A. Incorporation of amino acids into the protein of isolated mitochondria. A search for optimum conditions and a relationship to oxidative phosphorylation. Biochem J. 1962 Jun;83:588–596. doi: 10.1042/bj0830588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRUMAN D. E., LOEW H. EFFECTS OF SUBSTRATES FOR OXIDATIVE PHOSPHORYLATION AND OF RESPIRATORY INHIBITORS ON THE INCORPORATION OF AMINO ACIDS INTO PROTEIN BY ISOLATED BEEF-HEART MITOCHONDRIA. Exp Cell Res. 1963 Jun;31:230–232. doi: 10.1016/0014-4827(63)90179-4. [DOI] [PubMed] [Google Scholar]
- Truman D. E. The fractionation of proteins from ox-heart mitochondria labelled in vitro with radioactive amino acids. Biochem J. 1964 Apr;91(1):59–64. doi: 10.1042/bj0910059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von der Decken A., Campbell P. N. The effect of ultrasonic vibrations on the protein-synthesizing activity of microsome preparations from rat liver. Biochem J. 1964 Apr;91(1):195–201. doi: 10.1042/bj0910195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Haba G. L. INHIBITION BY PUROMYCIN OF AMINO ACID INCORPORATION INTO PROTEIN. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1721–1729. doi: 10.1073/pnas.45.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]


