Abstract
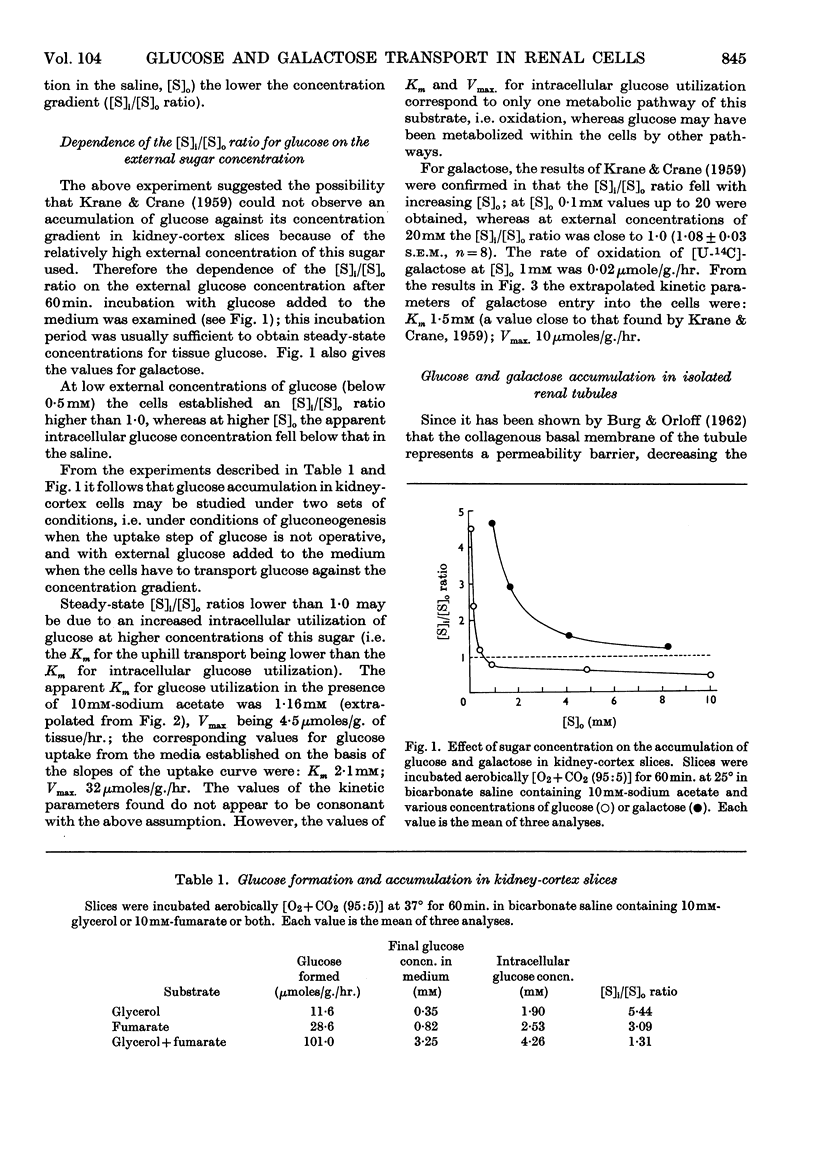
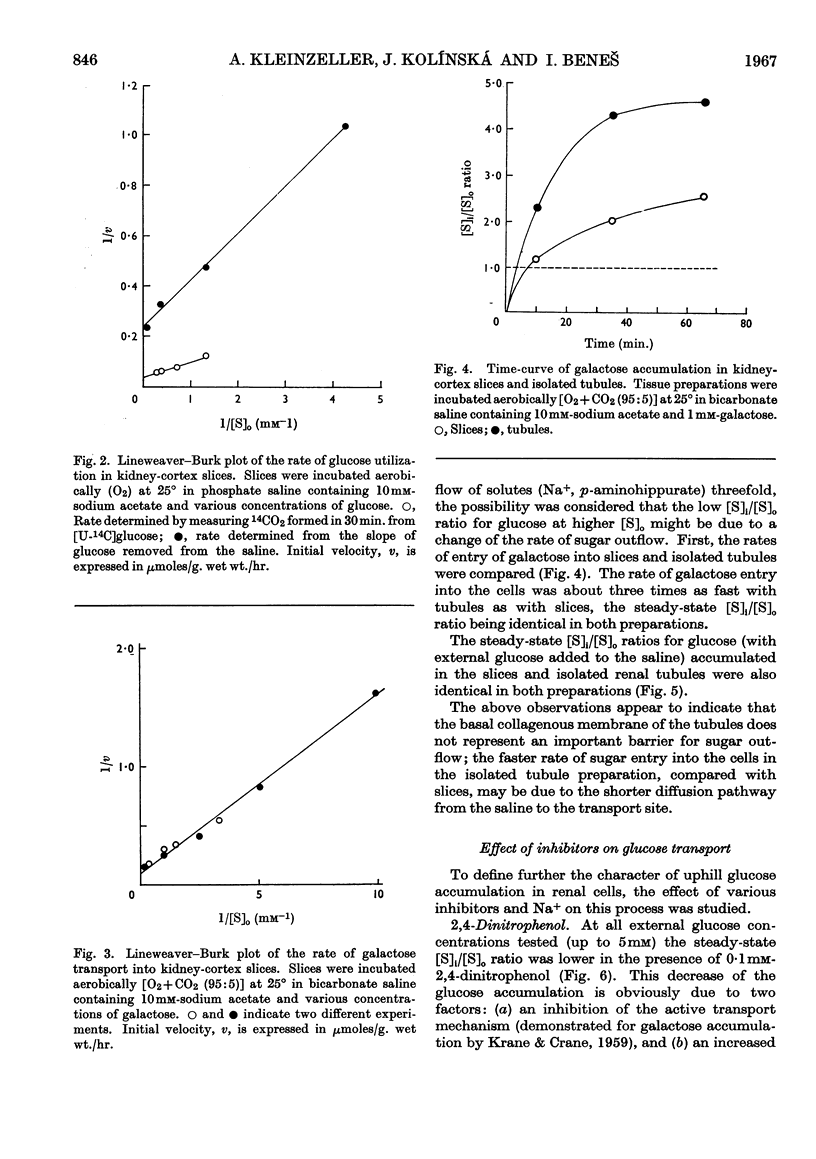
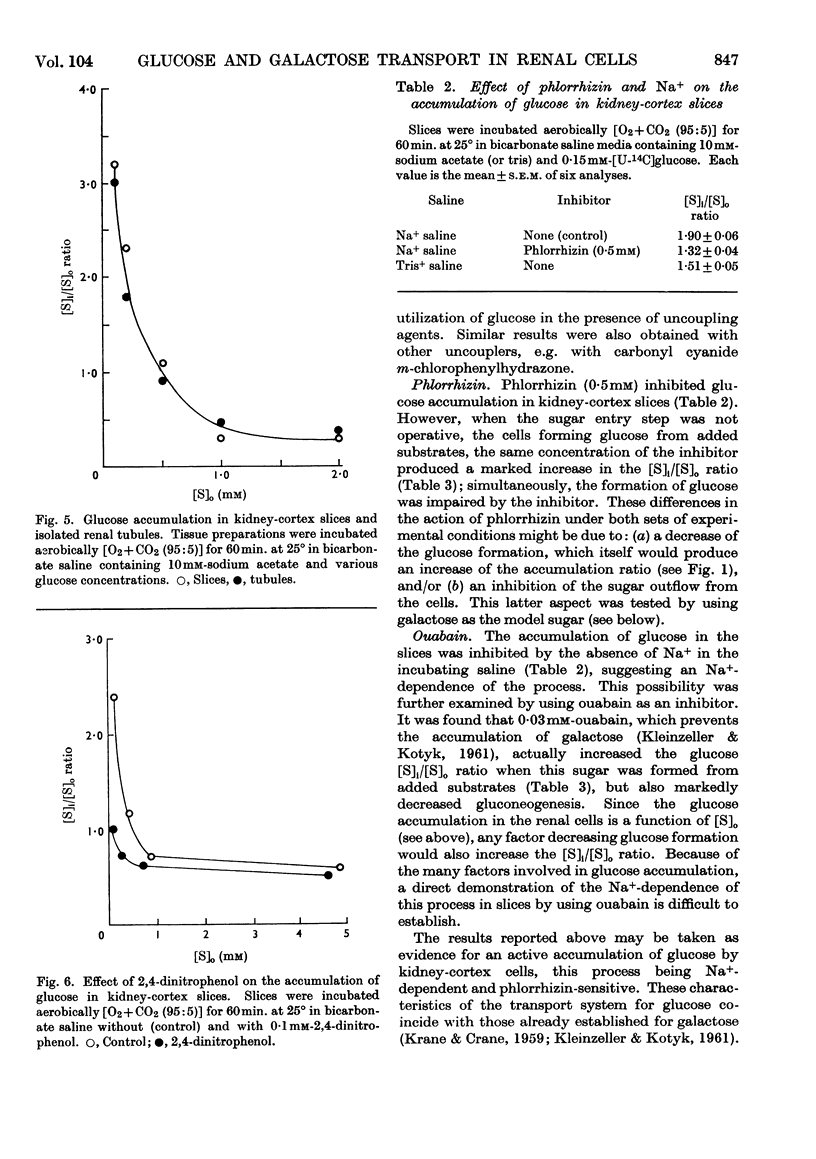
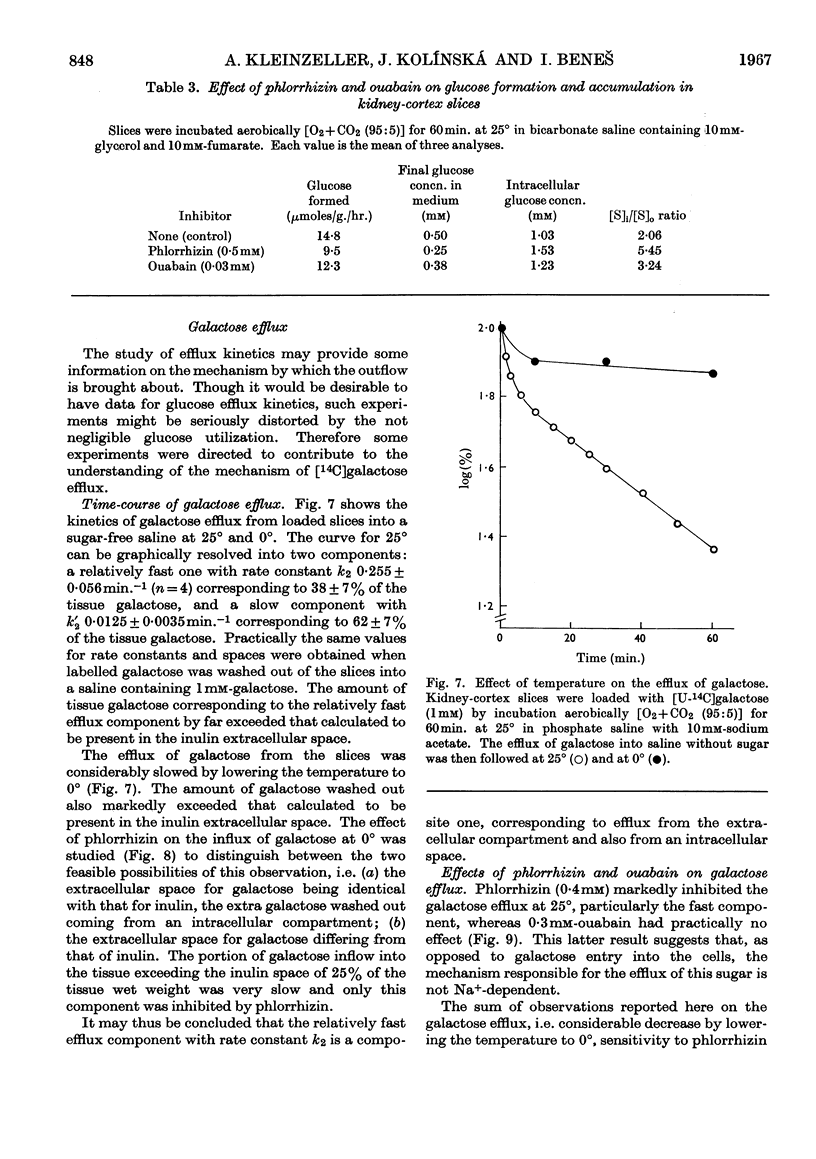
1. The aerobic transport of d-glucose and d-galactose in rabbit kidney tissue at 25° was studied. 2. In slices forming glucose from added substrates an accumulation of glucose against its concentration gradient was found. The apparent ratio of intracellular ([S]i) and extracellular ([S]o) glucose concentrations was increased by 0·4mm-phlorrhizin and 0·3mm-ouabain. 3. Slices and isolated renal tubules actively accumulated glucose from the saline; the apparent [S]i/[S]o fell below 1·0 only at [S]o higher than 0·5mm. 4. The rate of glucose oxidation by slices was characterized by the following parameters: Km 1·16mm; Vmax. 4·5μmoles/g. wet wt./hr. 5. The active accumulation of glucose from the saline was decreased by 0·1mm-2,4-dinitrophenol, 0·4mm-phlorrhizin and by the absence of external Na+. 6. The kinetic parameters of galactose entry into the cells were: Km 1·5mm; Vmax 10μmoles/g. wet wt./hr. 7. The efflux kinetics from slices indicated two intracellular compartments for d-galactose. The galactose efflux was greatly diminished at 0°, was inhibited by 0·4mm-phlorrhizin, but was insensitive to ouabain. 8. The following mechanism of glucose and galactose transport in renal tubular cells is suggested: (a) at the tubular membrane, these sugars are actively transported into the cells by a metabolically- and Na+-dependent phlorrhizin-sensitive mechanism; (b) at the basal cell membrane, these sugars are transported in accordance with their concentration gradient by a phlorrhizin-sensitive Na+-independent facilitated diffusion. The steady-state intracellular sugar concentration is determined by the kinetic parameters of active entry, passive outflow and intracellular utilization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado F. The relationship between Na+ and the active transport of arbutin in the small intestine. Biochim Biophys Acta. 1965 Nov 29;109(2):478–494. doi: 10.1016/0926-6585(65)90173-1. [DOI] [PubMed] [Google Scholar]
- BUHLER D. R. A simple scintillation counting technique for assaying C1402 in a Warburg flask. Anal Biochem. 1962 Nov;4:413–417. doi: 10.1016/0003-2697(62)90143-4. [DOI] [PubMed] [Google Scholar]
- BURG M. B., ORLOFF J. Oxygen consumption and active transport in separated renal tubules. Am J Physiol. 1962 Aug;203:327–330. doi: 10.1152/ajplegacy.1962.203.2.327. [DOI] [PubMed] [Google Scholar]
- Bosacková J., Crane R. K. Studies on the mechanism of intestinal absorption of sugars. 8. Cation inhibition of active sugar transport and 22Na influx into hamster small intestine, in vitro. Biochim Biophys Acta. 1965 Jul 22;102(2):423–435. doi: 10.1016/0926-6585(65)90132-9. [DOI] [PubMed] [Google Scholar]
- CSAKY T. Z., FERNALD G. W. Localization of the 'sugar pump' in the intestinal epithelium. Nature. 1961 Aug 12;191:709–710. doi: 10.1038/191709a0. [DOI] [PubMed] [Google Scholar]
- CSAKY T. Z., RIGOR B. M., Sr A CONCENTRATIVE MECHANISM FOR SUGARS IN THE CHOROID PLEXUS. Life Sci. 1964 Sep;3:931–936. doi: 10.1016/0024-3205(64)90101-8. [DOI] [PubMed] [Google Scholar]
- Clausen T. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. I. Stimulation of glycogen deposition and inhibition of lactic acid production in diaphragm, induced by ouabain. Biochim Biophys Acta. 1965 Sep 27;109(1):164–171. doi: 10.1016/0926-6585(65)90100-7. [DOI] [PubMed] [Google Scholar]
- Crane R. K. Na+ -dependent transport in the intestine and other animal tissues. Fed Proc. 1965 Sep-Oct;24(5):1000–1006. [PubMed] [Google Scholar]
- Curran P. F. Ion transport in intestine and its coupling to other transport processes. Fed Proc. 1965 Sep-Oct;24(5):993–999. [PubMed] [Google Scholar]
- Gordon E. E. Influence of ouabain on metabolism of rat kidney. Biochim Biophys Acta. 1965 Jul 8;104(2):606–608. doi: 10.1016/0304-4165(65)90370-3. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., CORI C. F. Studies of tissue permeability. V. The penetration and phosphorylation of 2-deoxyglucose in the rat diaphragm. J Biol Chem. 1959 Jan;234(1):171–177. [PubMed] [Google Scholar]
- KLEINZELLER A., JANACEK K., KNOTKOVA A. A simple method for measuring steady-state ion compartments and rate constants of ion fluxes in tissue slices. Biochim Biophys Acta. 1962 May 7;59:239–241. doi: 10.1016/0006-3002(62)90724-2. [DOI] [PubMed] [Google Scholar]
- KLEINZELLER A., KNOTKOVA A. THE EFFECT OF OUABAIN ON THE ELECTROLYTE AND WATER TRANSPORT IN KIDNEY CORTEX AND LIVER SLICES. J Physiol. 1964 Dec;175:172–192. doi: 10.1113/jphysiol.1964.sp007510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEINZELLER A., KOTYK A. Cations and transport of galactose in kidney-cortex slices. Biochim Biophys Acta. 1961 Dec 9;54:367–369. doi: 10.1016/0006-3002(61)90383-3. [DOI] [PubMed] [Google Scholar]
- KRANE S. M., CRANE R. K. The accumulation of D-galactose against a concentration gradient by slices of rabbit kidney cortex. J Biol Chem. 1959 Feb;234(2):211–216. [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. The use of glucose oxidase (notatin) for the determination of glucose in biological material and for the study of glucose-producing systems by manometric methods. Biochem J. 1948;42(2):230–238. [PMC free article] [PubMed] [Google Scholar]
- Kleinzeller A., Knotková A. Evaluation of the extracellular space of tissue slices from steady-state kinetics. Biochim Biophys Acta. 1966 Nov 8;126(3):604–605. doi: 10.1016/0926-6585(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Kleinzeller A., Kolínská J., Benes I. Transport of monosaccharides in kidney-cortex cells. Biochem J. 1967 Sep;104(3):852–860. doi: 10.1042/bj1040852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinzeller A. Oxidation of acetic acid in animal tissue. Biochem J. 1943;37(6):674–677. doi: 10.1042/bj0370674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinzeller A. The volume regulation in some animal cells. Arch Biol (Liege) 1965;76(2):217–232. [PubMed] [Google Scholar]
- Murthy L., Foulkes E. C. Movement of solutes across luminal cell membranes in kidney tubules of the rabbit. Nature. 1967 Jan 14;213(5072):180–181. doi: 10.1038/213180a0. [DOI] [PubMed] [Google Scholar]
- ROSENBERG L. E., DOWNING S. J., SEGAL S. Extracellular space estimation in rat kidney slices using C saccharides and phlorizin. Am J Physiol. 1962 Apr;202:800–804. doi: 10.1152/ajplegacy.1962.202.4.800. [DOI] [PubMed] [Google Scholar]
- Rosenberg T., Wilbrandt W. Carrier transport uphill. I. General. J Theor Biol. 1963 Sep;5(2):288–305. doi: 10.1016/0022-5193(63)90065-1. [DOI] [PubMed] [Google Scholar]
- SCHATZMANN H. J. Herzglykoside als Hemmstoffe für den aktiven Kalium- und Natriumtransport durch die Erythrocytenmembran. Helv Physiol Pharmacol Acta. 1953;11(4):346–354. [PubMed] [Google Scholar]
- Silverman M., Goresky C. A. A unified kinetic hypothesis of carrier mediated transport: its applications. Biophys J. 1965 Jul;5(4):487–509. doi: 10.1016/S0006-3495(65)86731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL G., LAUTERBACH F., KROEGER W. DIE BEDEUTUNG DES NATRIUMS FUER DIE RENALEN TRANSPORTE VON GLUCOSE UND PARA-AMINOHIPPURSAEURE. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 Mar 18;283:151–159. [PubMed] [Google Scholar]
- WHITTAM R. The permeability of kidney cortex to chloride. J Physiol. 1956 Mar 28;131(3):542–554. doi: 10.1113/jphysiol.1956.sp005481. [DOI] [PMC free article] [PubMed] [Google Scholar]


