Abstract
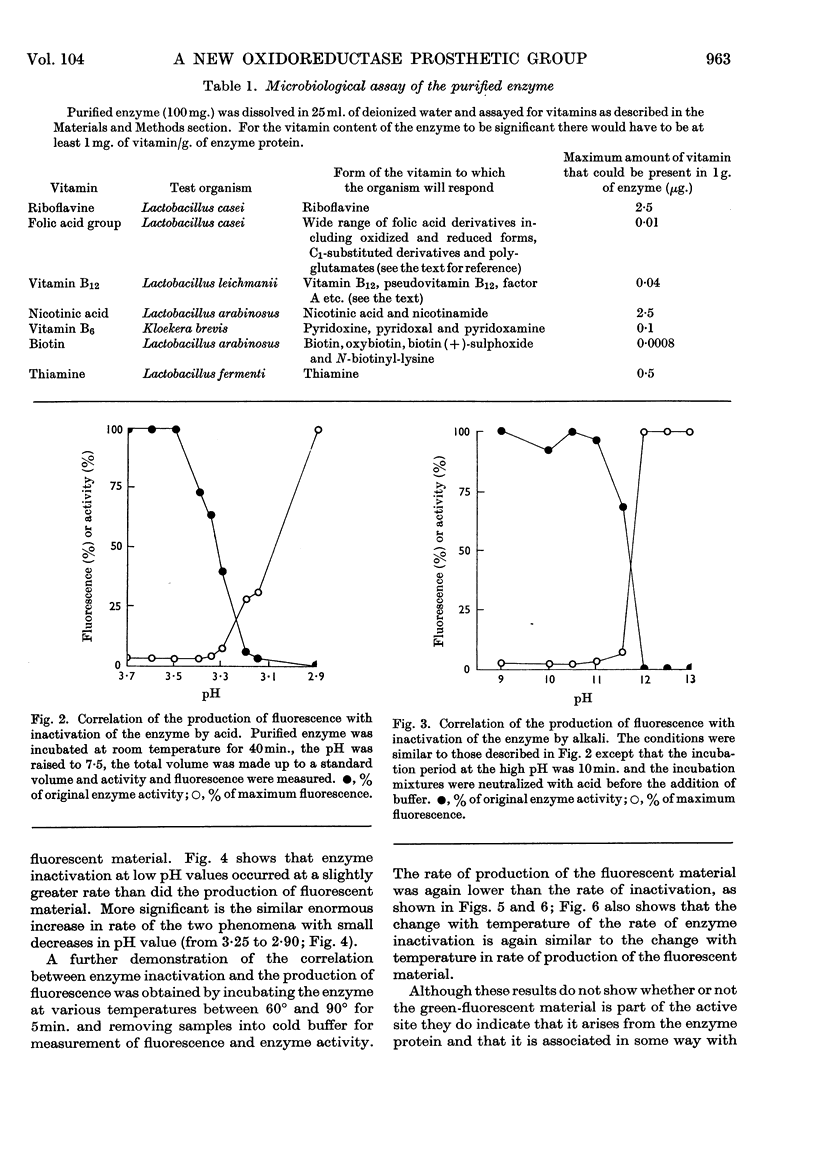
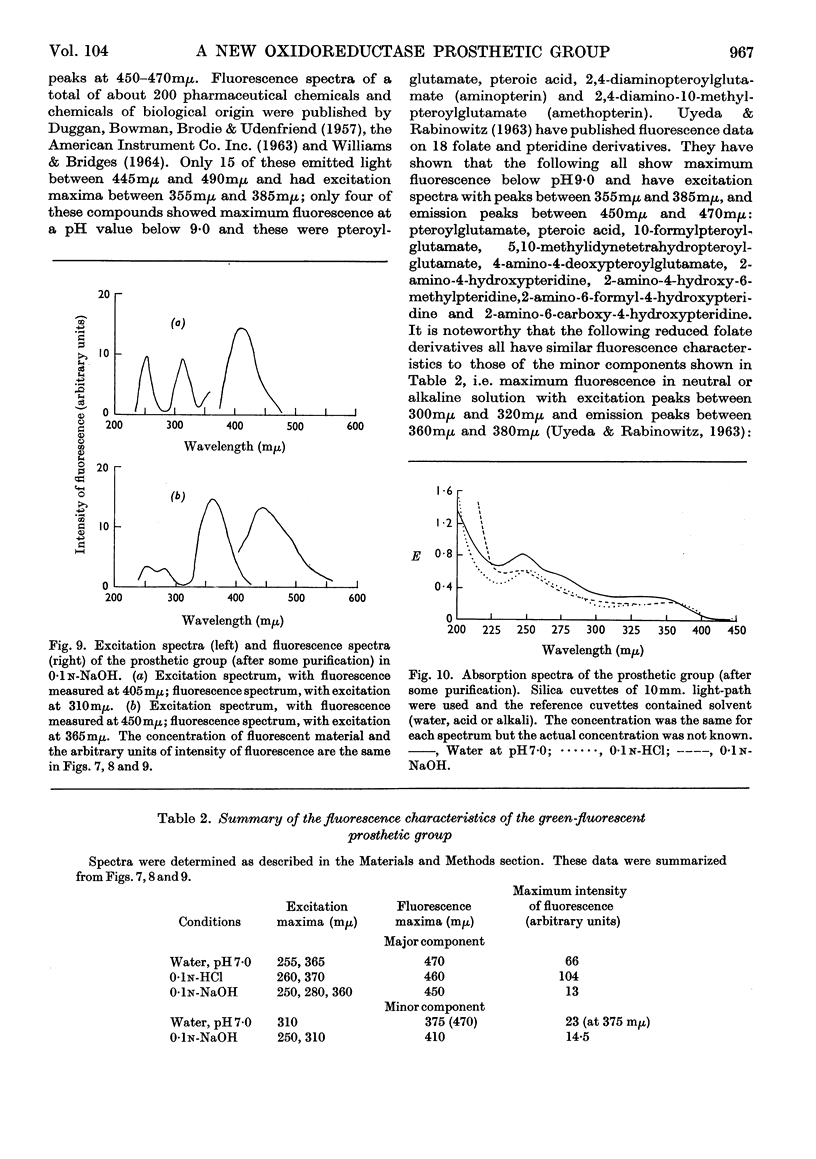
1. The purified alcohol dehydrogenase of Pseudomonas sp. M27, whose action is independent of nicotinamide nucleotides, has absorption peaks at 280mμ and at 350mμ with little or no absorption at or above 450mμ. 2. It does not fluoresce, but green-fluorescent material, diffusible on dialysis, is produced when the enzyme is treated with acid or alkali or when it is boiled. 3. Evidence is presented that the enzyme is not a flavoprotein. 4. Kinetic studies show a correlation between enzyme inactivation by acid, alkali or heat and liberation of the fluorescent material. 5. Some purification of the fluorescent material was achieved, but definite identification was not possible; the major component has a fluorescence maximum at about 460mμ with excitation maxima at about 260mμ and 365mμ. 6. Data are given (including absorption and fluorescence spectra) that support the suggestion that the prosthetic group of the enzyme is a pteridine derivative. 7. Possible mechanisms of action of the enzyme are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C., Zatman L. J. The microbial oxidation of methanol. 1. Isolation and properties of Pseudomonas sp. M27. Biochem J. 1964 Sep;92(3):609–614. doi: 10.1042/bj0920609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. 2. The methanol-oxidizing enzyme of Pseudomonas sp. M 27. Biochem J. 1964 Sep;92(3):614–621. doi: 10.1042/bj0920614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. Purification and properties of the alcohol dehydrogenase of Pseudomonas sp. M27. Biochem J. 1967 Sep;104(3):953–959. doi: 10.1042/bj1040953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C., Zatman L. J. The microbial oxidation of methanol. The alcohol dehydrogenase of Pseudomonas sp. M27. Biochem J. 1965 Sep;96(3):808–812. doi: 10.1042/bj0960808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT V. The assay and nature of folic acid activity in human serum. J Clin Invest. 1961 Jan;40:81–91. doi: 10.1172/JCI104240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMAN S. STUDIES ON THE STRUCTURE OF THE PRIMARY OXIDATION PRODUCT FORMED FROM TETRAHYDROPTERIDINES DURING PHENYLALAMINE HYDROXYLATION. J Biol Chem. 1964 Jan;239:332–338. [PubMed] [Google Scholar]
- Large P. J., Peel D., Quayle J. R. Microbial growth on C(1) compounds. 3. Distribution of radioactivity in metabolites of methanol-grown Pseudomonas AM1 after incubation with [C]methanol and [C]bicarbonate. Biochem J. 1962 Mar;82(3):483–488. doi: 10.1042/bj0820483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large P. J., Quayle J. R. Microbial growth on C(1) compounds. 5. Enzyme activities in extracts of Pseudomonas AM1. Biochem J. 1963 May;87(2):386–396. doi: 10.1042/bj0870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. I. Isolation and characterization of Pseudomonas AM 1. Biochem J. 1961 Dec;81:465–469. doi: 10.1042/bj0810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffo A., Testa E., Adinolfi A., Pelizza G., Moratti R. Control of the citric acid cycle by glyoxylate. Mechanism of the inhibition by oxalomalate and gamma-hydroxy-alpha-oxoglutarate. Biochem J. 1967 Apr;103(1):19–23. doi: 10.1042/bj1030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKEGGS H. R., NEPPLE H. M., VALENTIK K. A., HUFF J. W., WRIGHT L. D. Observations on the use of Lactobacillus leichmannii 4797 in the microbiological assay of vitamin B12. J Biol Chem. 1950 May;184(1):211–221. [PubMed] [Google Scholar]
- Shaw W. V., Tsai L., Stadtman E. R. The enzymatic synthesis of N-methylglutamic acid. J Biol Chem. 1966 Feb 25;241(4):935–945. [PubMed] [Google Scholar]
- TIETZ A., LINDBERG M., KENNEDY E. P. A NEW PTERIDINE-REQUIRING ENZYME SYSTEM FOR THE OXIDATION OF GLYCERYL ETHERS. J Biol Chem. 1964 Dec;239:4081–4090. [PubMed] [Google Scholar]
- UYEDA K., RABINOWITZ J. C. Fluorescence properties of tetrahydrofolate and related compounds. Anal Biochem. 1963 Jul;6:100–108. doi: 10.1016/0003-2697(63)90012-5. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. T., BRIDGES J. W. FLUORESCENCE OF SOLUTIONS: A REVIEW. J Clin Pathol. 1964 Jul;17:371–394. doi: 10.1136/jcp.17.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]


