Abstract
The NSF homolog Sec18 initiates fusion of yeast vacuoles by disassembling cis-SNARE complexes during priming. Sec18 is also required for palmitoylation of the fusion factor Vac8, although the acylation machinery has not been identified. Here we show that the SNARE Ykt6 mediates Vac8 palmitoylation and acts during a novel subreaction of vacuole fusion. This subreaction is controlled by a Sec17-independent function of Sec18. Our data indicate that Ykt6 presents Pal-CoA via its N-terminal longin domain to Vac8, while transfer to Vac8's SH4 domain occurs spontaneously and not enzymatically. The conservation of Ykt6 and its localization to several organelles suggest that its acyltransferase activity may also be required in other intracellular fusion events.
Keywords: palmitoylation, Sec18, vacuole fusion, Ykt6, SNARE
Introduction
The terminal step in membrane trafficking, fusion of lipid bilayers, is closely linked to the action of SNAREs (Rothman, 1994; Chen and Scheller, 2001; Rizo and Südhof, 2002; Jahn et al, 2003). These proteins have been implicated as either fusion catalysts or docking factors (Mayer, 1999). Little attention has been given to a possible role of SNAREs in the coordination of the fusion reaction.
Five SNAREs have been identified that are required for yeast homotypic vacuole fusion: the t- or Q-SNAREs Vam3, Vam7 and Vti1 and the v/R-SNAREs Nyv1 and Ykt6 (Nichols et al, 1997; Ungermann and Wickner, 1998; Ungermann et al, 1998a,Ungermann et al, 1998b,Ungermann et al, 1999) There is, however, a discrepancy. The analysis of SNARE complexes revealed that four coiled-coil domains form the core of the SNARE complex (Katz et al, 1998; Poirier et al, 1998; Sutton et al, 1998; Ungermann et al, 1999; Antonin et al, 2002). Thus, vacuole fusion requires one SNARE too many. In addition, the fusion of liposomes is efficient with the vacuolar t-SNAREs on one membrane and Nyv1 on the other membrane, but fails with Ykt6 as the sole v-SNARE (Fukuda et al, 2000). Ykt6 functioned as a v-SNARE in liposome fusion only after its farnesyl anchor had been replaced by a transmembrane domain (McNew et al, 2000). We decided to address this problem in detail.
Ykt6 is the most conserved SNARE in eucaryotes (Filippini et al, 2001; Tochio et al, 2001). It is required for trafficking to and within the Golgi (Lupashin et al, 1997; McNew et al, 1997; Tsui and Banfield, 2000; Zhang and Hong, 2002), for endocytic trafficking to the vacuole (Dilcher et al, 2001; Lewis and Pelham, 2002; Kweon et al, 2003) and for vacuole fusion (Ungermann et al, 1999). Ykt6 has a wide tissue distribution in rat and appears to have a specialized function in neuronal cells (Catchpoole and Hong, 1999; Hasegawa et al, 2003). It belongs to the longin class of v-SNAREs with a highly conserved N-terminal domain that suggests functionality (Filipini et al, 2001). Recently, the N-terminal domain of Ykt6 has been crystallized. It has a profilin-like fold very similar to the N-terminus of Sec22, a v/R-SNARE of the early secretory pathway (Gonzalez et al, 2001; Tochio et al, 2001). The interaction of the N-terminus with the SNARE domain suggests that Ykt6 is regulated in an autoinhibitory manner (Tochio et al, 2001).
The study of homotypic vacuole fusion allows the precise dissection of a fusion reaction. The reaction occurs in an ordered cascade of Sec18/17-dependent priming, a tethering reaction that requires the Rab GTPase Ypt7 and the HOPS/Class C Vps complex, SNARE-dependent docking and fusion. Several additional factors, like Ca2+/calmodulin and V0-ATPase, have been implicated in the last fusion step (reviewed by Mayer, 1999; Wickner, 2002). Vacuole fusion also requires palmitoylation of the fusion factor Vac8 (Veit et al, 2001; Wang et al, 2001).
Protein palmitoylation is a post-translational modification that is required for membrane fusion reactions (Glick and Rothman, 1987; Pfanner et al, 1990; Sakai et al, 2002), although the machinery has remained elusive. Myristoylated Vac8 has to be palmitoylated at three N-terminal cysteines to support vacuole fusion in vitro and in vivo (Wang et al, 1998,Wang et al, 2001; Schneiter et al, 2000; Veit et al, 2001). Vac8 palmitoylation occurs early in the fusion reaction and is Sec18-dependent, presumably to release Vac8 from an initial association with the cis-SNARE complex (Veit et al, 2001). It has been shown that palmitoylated Vac8 is required for fusion at a stage following trans-SNARE pairing (Wang et al, 2001). Recently, we presented evidence that acylation of Vac8 on vacuole membranes is enzyme-mediated, although the identity of the protein remained unknown (Veit et al, 2003).
We now show that Ykt6 has the acyltransferase activity for Vac8. The reaction is mediated by the N-terminal domain of Ykt6 that presents Pal-CoA to Vac8 and thus promotes acylation. On the vacuole, the Ykt6 activity requires an early function of Sec18 that is independent of its cofactors Sec17. The conservation of Ykt6 and Sec18 and their localization to several compartments within eucaryotic cells suggest that the same mechanism is responsible for fusion-dependent palmitoylation in higher eucaryotes.
Results
A requirement for Ykt6 in palmitoylation of Vac8
Sec18 disassembles cis-SNARE complexes and triggers Sec17 release from the vacuole (Ungermann et al, 1998a; Mayer, 1999). It is also necessary for Vac8 acylation, possibly by releasing cis-SNARE-associated Vac8 (Veit et al, 2001). To gain further insight into the mechanism by which Sec18 regulates Vac8's palmitoylation, we tested antibodies directed against components of the cis-SNARE complex for their ability to interfere with the acylation reaction. In contrast to antibodies to Sec18, those against Sec17 did not prevent palmitoylation (Figure 1A). However, the same antibodies to Sec17 block cis-SNARE complex disassembly and thus fusion at an early stage (Mayer et al, 1996; Ungermann et al, 1998a), suggesting that Vac8 acylation can occur independently of this reaction. This observation also indicates a function of Sec18 independent from Sec17. Second, of all antibodies to vacuolar SNARE proteins, only those to Ykt6 inhibited palmitoylation. Recombinant Ykt6 protein that contains a mutation at a conserved arginine (R71G) also prevented palmitoylation (Figure 1A) and interfered with the fusion reaction (Figure 1B). In contrast, the recombinant wild-type Ykt6 protein did not inhibit the fusion reaction (Figure 1C), suggesting that Ykt6-R71G competes against endogenous Ykt6 on vacuoles, thereby preventing fusion and palmitoylation. In agreement with the observation that palmitoylation is independent of SNARE complex disassembly, we found that Ykt6-R71G did not influence ATP-dependent Sec17 release from vacuoles (Figure 1D), an established assay to monitor priming (Mayer et al, 1996). Interestingly, all our antibodies to vacuolar SNAREs blocked Sec17 release, probably by masking the SNARE complex. Moreover, we found that excess recombinant Sec17 blocks Vac8 acylation, an effect that can be neutralized by adding exogenous Sec18 (Figure 1E). It was previously shown that excess Sec17 blocks vacuole fusion, but permits SNARE complex disassembly and release of endogenous Sec17 (Wang et al, 2000). Our data suggest that excess recombinant Sec17 competes for Sec18 that is required for palmitoylation, but not Sec18 attached to the SNARE complex. These results add to the picture that Sec18 together with Ykt6 performs a novel function in palmitoylation unrelated to Sec17-dependent disassembly of cis-SNARE complexes.
Figure 1.
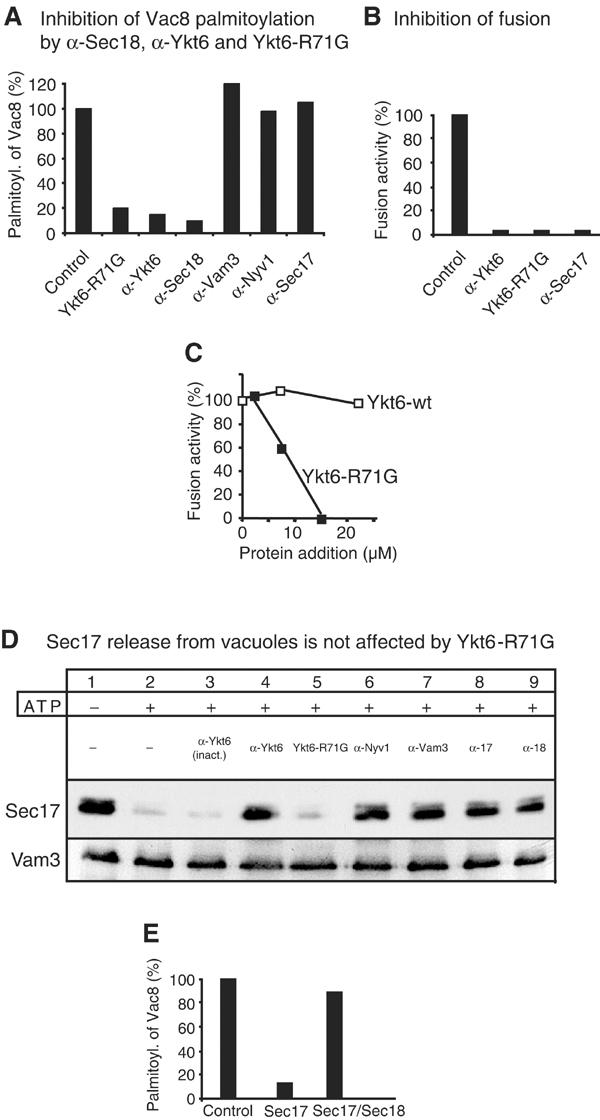
Ykt6 is implicated in a Sec17-independent early reaction. (A) Palmitoylation of Vac8 is specifically inhibited by antibodies to Ykt6 and Sec18. Fusion reactions (300 μl) containing 60 μg of vacuoles from BJ3505 were incubated for 10 min at 26°C in the presence of ATP (0.5 mM), cytosol (0.5 μg/μl), coenzyme A (CoA, 10 μM), [3H]-palmitate (150 μCi), and either purified IgGs to the indicated proteins or recombinant Ykt6-R71G (15 μM) or an equal volume of PS buffer (20 mM PIPES/KOH, 200 mM sorbitol). Vacuoles were then isolated by centrifugation (10 min, 12 000 g, 4°C), washed with 500 μl of PSK buffer (20 mM PIPES/KOH, pH 6.8, 200 mM sorbitol, 150 mM KCl) and resuspended in SDS sample buffer without 2-mercaptoethanol. Palmitoylated Vac8 was identified by SDS–PAGE and fluorography. Bands were quantified by laser densitometry (IPLab GelH, Scientific Image Processing 1.5, Signal Analytics). The positive control was set to 100%. (B) Interference with Ykt6 blocks fusion. Standard fusion reactions were incubated for 60 min at 26°C in the presence of Ykt6-R71G (15 μM) or anti-Ykt6 antibodies. The inhibiting amounts of protein were determined by titration (see Figure 1C). Background activity was subtracted from all measurements. Fusion activity in the absence of inhibitor was set to 100%. (C) Inhibition of fusion by Ykt6-R71G, but not Ykt6-wt. Indicated amounts of purified Ykt6 proteins were added to standard fusion reactions. Alkaline phosphatase activity was determined after 90 min at 26°C. (D) Sec17 release is unaffected by Ykt6-R71G. BJ3505 vacuoles were incubated for 10 min at 26°C in a 150 μl reaction containing 200 ng/ml Sec18 in the presence or absence of Ykt6-R71G (15 μM), anti-Ykt6 (inactive), anti-Ykt6, anti-Vam3, anti-Nyv1, anti-Sec17 or anti-Sec18. Then vacuoles were placed on ice, centrifuged (5 min, 16 000 g, 4°C) and washed twice with 500 μl PSK buffer. Vacuoles were analyzed by immunoblotting with anti-Sec17 and anti-Vam3 antibodies. (E) Excess recombinant Sec17 blocks palmitoylation. Palmitoylation reactions were performed as in (A). Recombinant Sec17 (7 μmol) or Sec18 (12.5 μmol) was added where indicated. At the same concentration, Sec17 inhibited the fusion reaction completely (data not shown), in agreement with previous studies (Wang et al, 2000).
The longin domain of Ykt6 is involved in acylation and Pal-CoA binding
A role of Ykt6 in the acylation reaction was unexpected. Ykt6 is the most conserved SNARE, and bound to membranes via an isoprenoid anchor (Chen and Scheller, 2001; Tochio et al, 2001). It is required for multiple membrane fusion reactions along the secretory pathway (Lupashin et al, 1997; McNew et al, 1997; Dilcher et al, 2001) and to the yeast vacuole (Kweon et al, 2003). Because of its conserved extended N-terminal domain, Ykt6 has been classified as a member of the longin family (Filippini et al, 2001). This domain is characterized by a profilin-like fold that can interact with the C-terminal SNARE domain in an autoinhibitory manner (Tochio et al, 2001). To investigate whether this domain is involved in regulating palmitoylation of Vac8, we generated a recombinant Ykt6 N-terminal peptide (amino acids 1–140; Tochio et al, 2001) and antibodies to it. Strikingly, both were sufficient to inhibit vacuole fusion (Figure 2A) and Vac8 palmitoylation (Figure 2B), indicating a direct involvement of the N-terminal domain. We speculate that the Ykt6 N-terminal peptide inhibits vacuole fusion due to the lack of a C-terminal coiled-coil domain. Thereby, it might act as a competitor for components that are required for palmitoylation and fusion. Furthermore, a Psi-BLAST analysis revealed a short, but significant alignment of the N-terminal region of Ykt6 with the β-ketoacyl synthase subunit of fatty acid synthase (Figure 2C). This activity transfers C2-fragments from malonyl-CoA to the growing acyl chain. The homology region shown does not include histidine 292 of the fatty acid synthase that has been implicated in catalysis (Witkowski et al, 2002). We therefore speculated that the N-terminal domain of Ykt6 could be involved in fatty acid or CoA binding. Since we found that Vac8 and Ykt6 are proximal to each other on vacuoles (Figure 2D), we asked whether Ykt6 or its N-terminal domain could bind to palmitoyl-CoA or CoA, thus serving as an acceptor of activated palmitate during the acylation reaction. Full-length Ykt6 and the N-terminal fragment bind [3H]-Pal-CoA efficiently when compared to a control fatty acid binding protein, bovine serum albumin (BSA; Figure 2E). Furthermore, when we used [3H]-acetyl-CoA as a substrate, BSA did not bind, while Ykt6-R71G or just the N-terminus still did (Figure 2F). Ykt6-wt behaved like Ykt6-R71G in the binding assays (not shown). A control protein, Vti1-GST, did not bind to Pal-CoA, and heat inactivation of Ykt6 abolished binding completely (not shown). Preliminary experiments show that Ykt6 is also able to recognize palmitate specifically (not shown). This suggests that Ykt6 binds both the fatty acid and the CoA moiety, and therefore provides specificity for the transfer. Binding of Ykt6 to Pal-CoA or CoA is, however, not strong under these conditions since we required an excess of Ykt6 proteins to see efficient binding. These data suggest that the N-terminus of Ykt6 might itself support the acylation of Vac8 by directly interacting with the activated fatty acid. The N-terminus is indeed important for Ykt6 function within yeast. Mutants lacking the N-terminal domain of Ykt6 or deleted in 30 amino acids of the conserved region (residues 60–90) are not viable (Figure 2G). Thus, our results point toward a new function of the N-terminal longin domain.
Figure 2.
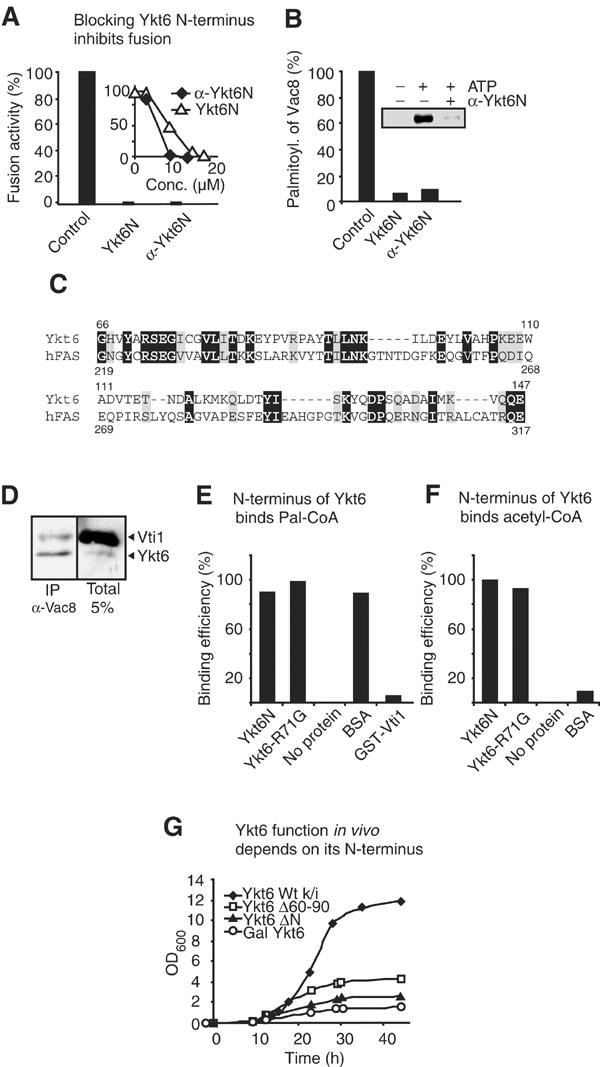
Essential function of the N-terminal domain of Ykt6. (A) Inhibition of fusion by the N-terminal domain. Fusion reactions were performed as described in Materials and methods in the presence of antibodies to Ykt6N or recombinant Ykt6N (22 μM) according to the titration (see the inset). (B) Inhibition of palmitoylation by N-terminal antibodies or recombinant Ykt6N. Palmitoylation in the absence or presence of Ykt6N (22 μM) or anti-Ykt6N was performed as in Figure 1A. (C) Sequence alignment of Ykt6 (accession number P36015) and human fatty acid synthase (P49327) as revealed by Psi-BLAST. The alignment of homologous regions is shown. Identical residues are in black and conserved ones in gray (32% identical, 49% conserved). (D) Ykt6 is proximal to its substrate Vac8 on vacuoles. Isolated BJ3505 vacuoles (60 μg) were incubated with 200 μM of the cleavable crosslinker DSP for 30 min on ice. Vacuoles were then reisolated, lysed in 10 μl 10% SDS, boiled for 5 min at 95°C and then detergent solubilized in 1 ml IP buffer (1% Triton X-100, 300 mM NaCl, 10 mM Tris/HCl, pH 7.4). Immunoprecipitation and analysis was carried out with protein A-coupled antibodies to Vac8 as described (Veit et al, 2001). Immunoblots were decorated with antibodies to Vti1 and Ykt6. (E,F) The N-terminal domain of Ykt6 binds to CoA. [3H]-Pal-CoA (1.7 fmol (0.1 μCi); American Radiolabeled Chemicals, Inc. (E)) or [3H]-acetyl-CoA (same concentration (F)) was incubated alone or together with 20 μg of the indicated protein in 500 μl PS buffer at room temperature for 10 min. Then, a piece of nitrocellulose (1 cm2; Protran, Schleicher and Schuell) was added to the reaction. After 30 min, the nitrocellulose was washed three times for 10 min in PSK buffer. The amount of [3H]-Pal-CoA/[3H]-acetyl-CoA attached to the nitrocellulose was determined by scintillation counting. Unspecific binding of Pal-CoA/acetyl-CoA to nitrocellulose (no protein) was subtracted and set to 0% (CoA: 0%=30 cpm, 100%=170 cpm; Pal-CoA: 0%=2800 cpm, 100%=5000 cpm). The signal obtained for BSA, which binds at least six acyl chains per protein, was divided by six in (E) and set to 100%. BSA was not able to bind to acetyl-CoA (F). (G) Growth kinetics. BJ Gal-Ykt6 (full-length) strains containing chromosomally integrated pRS406 plasmids encoding full-length Ykt6, a deletion mutant lacking amino acids 60–90 (Ykt6Δ60–90) or one lacking the N-terminus (Ykt6ΔN) were grown overnight in galactose-containing YP medium, and then diluted to an OD600 of 0.002 in fresh YP medium containing glucose in order to switch off the expression of Gal-Ykt6. The cultures were incubated at 30°C and OD600 was determined at the indicated time points.
The N-terminus of Ykt6 is required early in fusion
Ykt6 is known to be involved in a docking-related stage of vacuole fusion (Ungermann et al, 1999). Our results indicate an unexpected early function of Ykt6. To address this seeming contradiction, we performed kinetic studies of the fusion reaction in order to determine the stage at which the N- and C-termini of Ykt6 function (Figure 3). The last step, at which an inhibitor acts, can be determined by adding the inhibitor at various time points to an ongoing fusion reaction and monitoring when resistance against the inhibitor is gained. The priming inhibitors anti-Sec17 and anti-Sec18 block fusion only within the first 10 min, whereas inhibitors of docking like anti-Vam3 do so until about 30 min (Mayer et al, 1996). The tethering inhibitor Gdi1, which extracts Ypt7, inhibits after priming, but before docking (not shown). An aliquot set on ice at each time point shows the progression of the fusion reaction until that time. This assay allows a distinction between different curves that represent stages of the fusion reaction. Due to variations in vacuole preparations, the kinetics of the reaction may change, but not the order of the curves. Antibodies to full-length Ykt6 block the reaction slightly before docking, as did recombinant Ykt6-R71G or just the C-terminal part (Figure 3A,B, data not shown), similar to the tethering inhibitor Gdi1 (not shown). In contrast, the N-terminal fragment of Ykt6 or antibodies to the Ykt6 N-terminus only inhibited when added during the first 15 min of the reaction, that is, during priming (Figure 3C, D). This confirms an early function of the N-terminus and correlates with the kinetics of Vac8's palmitoylation (Veit et al, 2001). The C-terminal part of Ykt6 appears to function at tethering and not at docking (trans-SNARE pairing) like the other antibodies to vacuolar SNAREs (Ungermann et al, 1998a,Ungermann et al, 1999), a function that we are now exploring.
Figure 3.
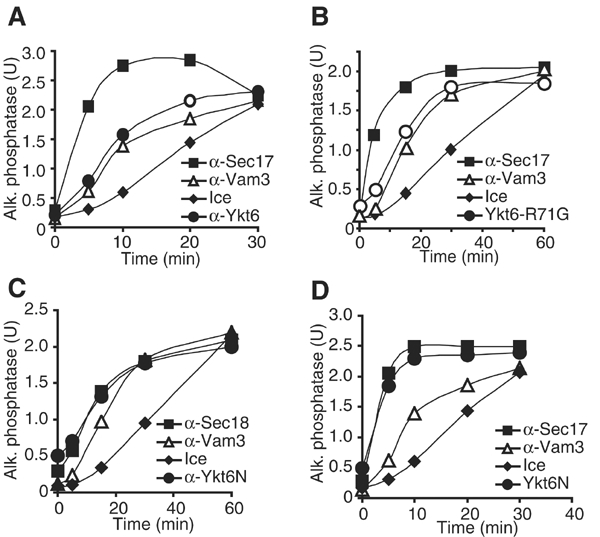
Ykt6 is involved in early and later steps of fusion. A 30 × scale fusion reaction was started in the presence of 0.5 mM ATP and 10 μM CoA at 26°C. Aliquots (30 μl) were removed at the indicated times, added to recombinant Ykt6-R71G, an N-terminal peptide of Ykt6 (Ykt6N) or antibodies to Ykt6, Ykt6N, Sec17, Sec18 or Vam3, and incubated at 26°C or placed on ice. Reactions were incubated for a total of 90 min before assaying for alkaline phosphatase activity. Inhibition by anti-Ykt6 (A), Ykt6-R71G (B), anti-Ykt6N (C) and Ykt6N (D).
The vacuolar acyltransferase activity co-migrates with Ykt6
Our data are so far consistent with a role of both Ykt6 and Sec18 during palmitoylation. Moreover, the N-terminal domain of Ykt6 appears to have a direct influence on palmitoylation. To investigate how direct the involvement of both proteins is, we took advantage of our recent observations that palmitoylation activity can be recovered from a vacuolar detergent extract by using activated palmitate ([3H]-Pal-CoA) as a substrate (Veit et al, 2003). Incubation of the detergent extract with recombinant Vac8, but not with a mutant lacking the N-terminal cysteines, results in efficient palmitoylation (Veit et al, 2003). To characterize the palmitoylation activity in more detail, we fractionated the extract in a glycerol gradient and analyzed the fractions for acylation activity (Figure 4). Palmitoylation efficiency of recombinant Vac8 protein peaked in fractions #2–3, at an estimated molecular weight of 15–40 kDa, and was suppressed by an antibody to Ykt6 (not shown). Ykt6 was recovered in the same fractions as the acylation activity, whereas Sec18 had its peak in fractions #4–6 at its molecular weight of 80 kDa. Thus, our data suggest that Sec18 is not required for palmitoylation. Ykt6 alone could mediate the reaction and not as part of a large protein complex.
Figure 4.
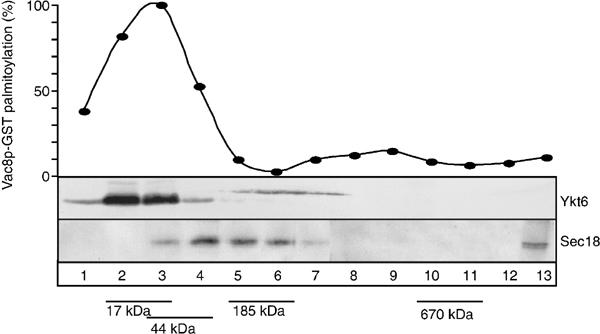
Acyltransferase activity in vitro corresponds to Ykt6. Sizing of the acyltransferase activity. Vacuoles (60 μg) were preincubated in PSK buffer for 10 min at 26°C without ATP, reisolated and solubilized in 300 μl 0.1% Triton X-100, 20 mM Tris/HCl (pH 7.4) and 150 mM NaCl for 10 min on ice. Unsolubilized material was removed by centrifugation (10 min, 14 000 × g, 4°C). The cleared detergent extract was loaded on top of a 10.5 ml 10–34% continuous glycerol gradient in PSK buffer and centrifuged (SW41, 40 000 g, 4°C, 18 h). Equal fractions (750 μl) were collected from the top of the gradient. Aliquots of the fractionated extract (10 μl) were collected and analyzed. To determine PAT activity, 10 μl extract and recombinant myr-Vac8-GST were incubated for 30 min at 26°C in a 100 μl reaction in the presence of [3H]-Pal-CoA (Pal-CoA) and then processed by SDS–PAGE of precipitated proteins. Fluorographs were quantified by laser densitometry. Aliquots of the same fraction were resolved on SDS–PAGE gels and blotted onto nitrocellulose to analyze the size of Sec18 and Ykt6 in the gradient. The distribution of Sec18 and Ykt6 is shown. For size determination, a control gradient with marker proteins was run in parallel. Sizes are indicated.
Transfer of Pal-CoA to Vac8 in vitro depends on Ykt6
We directly tested this hypothesis by incubating recombinant Vac8-GST with purified Ykt6 proteins or Sec18 in the presence of [3H]-Pal-CoA (Figure 5A). Indeed, addition of Ykt6-wt alone was sufficient for in vitro acylation of Vac8-GST (lane 2). In contrast, Sec18 had no acyltransferase activity (lane 3), and did not stimulate Ykt6-mediated acylation significantly (not shown). The N-terminal domain of YKt6 alone, fused to GST, was sufficient for palmitoylation, while the N-terminus of Vti1 as a GST fusion protein was not. This implies that the acylation activity is confined to the N-terminus of Ykt6 as suggested by our deletion and antibody analysis (Figure 1A,2G). The Ykt6-mediated acylation was specific, since heat inactivation of the protein abolished labeling of Vac8-GST. Thus, the N-terminal domain of Ykt6 is sufficient for acylation of Vac8, providing an unexpected connection between a reversible protein lipidation and SNARE function. Sec18 is not necessary for palmitoylation, but may provide an important regulatory function on the vacuole.
Figure 5.
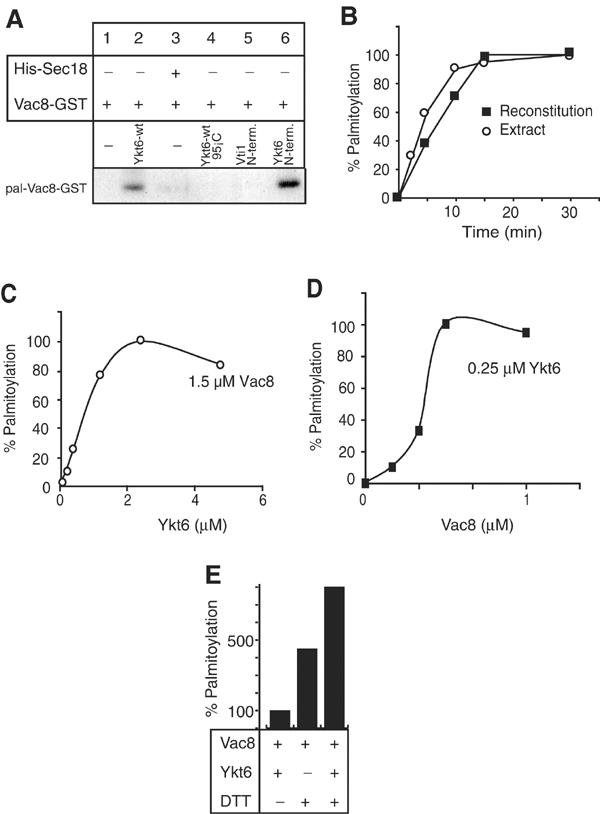
Ykt6 is sufficient for Vac8 palmitoylation. (A) In vitro reconstitution of the acyltransferase reaction depends on Ykt6. Indicated combinations of recombinant Vac8-GST (0.5 μM) and Ykt6-wt (2 μM) were incubated with 0.5 μM [3H]-Pal-CoA for 60 min at 26°C in the buffer of the standard fusion reaction. For inactivation, Ykt6 was heated for 5 min at 95°C before being added to the assay. In lanes 5 and 6, Ykt6(1–120)-GST (Ykt6 N-term.) or GST-Vti1(1–110) (Vti1-N-term.) were used instead of Ykt6-wt. After the assay, proteins were precipitated by chloroform/methanol, dried, resuspended in SDS sample buffer, resolved on SDS–PAGE and analyzed by fluorography. (B) Kinetics of the in vitro acylation reaction. Reactions were performed as described in (A). Either purified Ykt6 or a vacuolar lysate (Figure 4A) was used as the enzyme source. Fluorograms were quantified by laser densitometry. Maximal palmitoylation observed in the reaction was set to 100%. (C) Determination of the stoichiometry of the reaction. Increasing amounts of Ykt6 were titrated against 1.5 μM Vac8. The reaction was performed and analyzed as described in (A). (D) Indicated amounts of Vac8 were titrated against 0.25 μM Ykt6. (E) Ykt6 stimulates acylation under reducing conditions. Acylation was performed as in (A). Where indicated, 1 mM DTT was added to the reaction. Ykt6-mediated palmitoylation in the absence of DTT was set as 100%.
To characterize the mechanism by which Ykt6 palmitoylates Vac8, we measured stoichiometry and kinetics of the in vitro palmitoylation reaction. Acylation of endogenous Vac8 on vacuoles occurs within a few minutes, indicating that Ykt6 is positioned such that it acylates Vac8 immediately (LEP Dietrich, M Cristodero and C Ungermann, unpublished; see Figure 2D). A vacuolar detergent extract used as an enzyme source palmitoylates recombinant Vac8-GST, although the reaction requires 20–30 min until completion (Veit et al, 2003; Figure 5B). Consistently, Ykt6-mediated in vitro acylation of Vac8-GST has comparable kinetics (Figure 5B). To determine stoichiometry, we titrated Ykt6 into a reaction with a defined concentration of Vac8-GST (1.5 μM; Figure 5C). (Micromolar amounts of Vac8-GST are required to detect a palmitoylation signal, representing a technical limitation of the assay (our unpublished observations).) Interestingly, we observed that the reaction saturated at approximately equimolar concentrations of Ykt6 and Vac8, while limiting Ykt6 amounts were insufficient for in vitro acylation (Figure 5C). To confirm this, we did the converse experiment, and titrated Vac8-GST into the acylation reaction while keeping Ykt6 constant (Figure 5D). Indeed, at approximately equimolar amounts of Vac8 and Ykt6 acylation was most efficient, while increasing the Vac8 concentration did not result in more acylation. Accordingly, with a five-fold higher Ykt6 amount, approximately five-fold more Vac8 could be modified (not shown). This confirms that similar micromolar amounts of Ykt6 and Vac8 are required for efficient in vitro acylation. The ratio of Vac8 to Ykt6 and the slow kinetics make it unlikely that Ykt6 is enzymatically involved in Vac8 palmitolyation. Based on this and Ykt6's ability to bind Pal-CoA, we propose that Ykt6 serves acylation by specifically presenting activated palmitate to Vac8. How is the acylation itself mediated? A feature of most palmitoylated proteins is that in vitro they become acylated at the authentic cysteines independently of any enzymatic protein source (Duncan and Gilman, 1996; Bano et al, 1998; Veit, 2000; Bizzozero et al, 2001). In Figure 5E we show that the same is true for Vac8 if the reducing environment of the cytosol is simulated by adding 1 mM of the reducing agent DTT (lane 2), and is stronger than Ykt6-mediated acylation in the absence of DTT (lane 1). This indicates that in the presence of abundant activated palmitate (Pal-CoA), palmitoylation can occur spontaneously in the reducing atmosphere of the cytosol. However, within the cell, the pool of free Pal-CoA is too small (in the nanomolar range) to allow the reaction to take place (Faergeman and Knudsen, 1997). A possible mechanism for protein palmitoylation is that protein-bound Pal-CoA is specifically targeted to its substrate, followed by spontaneous thioesterification. Indeed, addition of Ykt6 to the reaction in the presence of DTT increases Vac8 acylation synergistically (lane 3), implying that Ykt6 is able to stimulate the transfer also under reducing conditions. Our data thus indicate that Ykt6 is the protein presenting Pal-CoA to Vac8. This model is very attractive since it would bring together the conflicting results on the mechanism of protein acylation that were published over the last 20 years. Our model agrees with the assumption that thioesterification between palmitate and protein occurs spontaneously (Bano et al, 1998; Bizzozero et al, 2001; Qanbar and Bouvier, 2003). But it also demonstrates the need for a specific protein that enables and controls this reaction in vivo. It should be noted that none of the recently published palmitoyl transferases were demonstrated to act enzymatically (Lobo et al, 2002; Roth et al, 2002), and that Ykt6-mediated palmitoylation of Vac8 occurs with similar efficiency when we used the buffer conditions described by Lobo et al (not shown). In agreement with our results, similar amounts of PAT and substrate are required in all cases.
Ykt6-mediated acylation of Vac8 occurs in a novel subreaction of vacuole fusion
Finally, we directly tested the idea that Ykt6 and Sec18 participate together in a mechanism that is independent of cis-SNARE disassembly. When fusion reactions were incubated in the presence of antibodies to Sec18 to inhibit priming, then reisolated and resuspended in the presence of purified Sec18 protein to reverse the inhibition, reactions were still sensitive to Ykt6-R71G and to antibodies to Sec17, Vam3 and Vam2, a subunit of the HOPS/Class C Vps tethering complex (Price et al, 2000; Seals et al, 2000; Wurmser et al, 2000). In contrast, if recombinant Ykt6-R71G was applied in a first incubation in the presence of ATP and removed later by re-isolating the vacuoles, reactions were still sensitive to antibodies to Sec18 (Figure 6B). While remaining sensitive to anti-Sec18, though, the reaction gained resistance to anti-Sec17. Resistance was also gained to anti-Vam2 and anti-Vam3, suggesting that a block in Ykt6 function allows tethering and docking, even though it prevents fusion of yeast vacuoles. This nicely fits the observation that a block in palmitoylation of Vac8 allows trans-SNARE pairing, but prevents fusion (Wang et al, 2001). Thus, acylation mediated by Sec18 and Ykt6 is triggered independently of the established priming reaction and marks a novel pathway in vacuole fusion.
Figure 6.
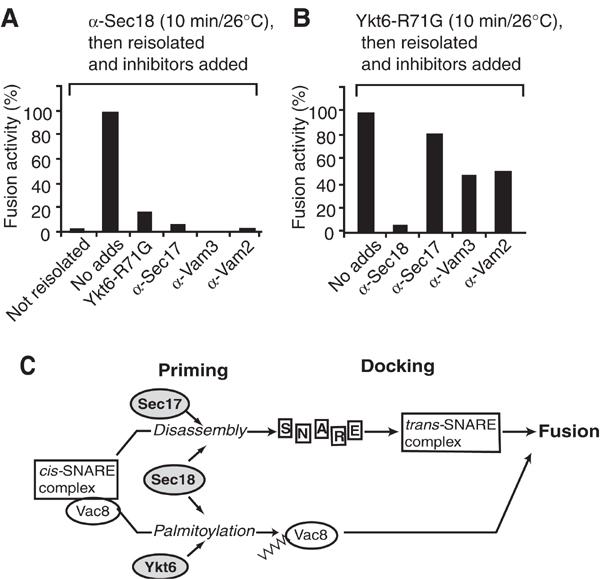
Role of Sec18 beyond Sec17-dependent priming. (A) Vacuoles were incubated with anti-Sec18 antibody in a standard fusion reaction with cytosol at 26°C. After 10 min, one sample (no reisolation) was set aside, and the others were diluted 10-fold in PSK buffer, centrifuged (5 min, 9000 g, 4°C) and resuspended in the original volume of reaction buffer with cytosol and 0.2 μg Sec18, containing the indicated inhibitors. After an additional 60 min at 26°C, alkaline phosphatase activity was determined. Background activity was subtracted. The activity of control samples (no adds) was set to 100%. (B) His-Ykt6 was added instead of anti-Sec18 to vacuoles, and incubations and reisolation were performed as before. (C) Working model of vacuole fusion. For details, see text.
Discussion
Our data reveal several surprising new insights into fusion-dependent palmitoylation. First, we have shown that Sec18 is linked to Ykt6-mediated palmitoylation in a mechanism independent of Sec17. This novel subreaction of vacuole fusion is initiated in parallel to the established priming and docking reaction. Second, we have identified and characterized the SNARE Ykt6 as the factor responsible for palmitoylation of Vac8. Our data suggest that the N-terminal domain of Ykt6 binds activated palmitate and promotes its transfer to Vac8. This reaction appears to proceed by an unexpected chaperone-like mechanism.
Our data suggest the following model (Figure 6C). At priming, Sec18 fulfills two functions: (a) together with Sec17 it causes cis-SNARE disassembly (Mayer et al, 1996; Ungermann et al, 1998a), and (b) together with Ykt6 it is required for palmitoylation of Vac8. Palmitoylated Vac8 then functions at a step following trans-SNARE complex formation (Wang et al, 2001). This observation extends previous pictures of the priming reaction (Wickner, 2002), now demonstrating that multiple, parallel reactions are initiated at this stage.
A function of Sec18 or NSF has previously been associated with its ability to bind to the SNARE complex (Wilson et al, 1992; Hanson et al, 1997; Hohl et al, 1998), and trigger its disassembly via ATP hydrolysis (Söllner et al, 1993). But there are data that suggest that Sec18/NSF has multiple roles: NSF, together with its cofactor α-SNAP, has a role distinct from ATP-dependent SNARE complex disassembly by catalyzing the association of GATE-16 with the v-SNARE GOS-28 in the re-formation of the postmitotic Golgi (Müller et al, 1999,Müller et al, 2002). NSF is also required with α-SNAP to activate the glutamate receptor at the postsynaptic membrane. The activation of the glutamate receptor is unrelated to the conventional role of NSF in fusion reactions (Nishimune et al, 1998; Osten et al, 1998; Song et al, 1998; Hanley et al, 2002). We do not yet know how Sec18p regulates palmitoylation, a focus that will be the subject of a separate manuscript (LEP Dietrich and C Ungermann, in preparation).
The identification of a novel function of Ykt6 in addition to its role as a SNARE is of major importance. It has been puzzling why vacuole fusion requires five SNAREs (Ungermann et al, 1999) even though the core complex contains only four helices (Katz et al, 1998; Poirier et al, 1998; Sutton et al, 1998; Ungermann et al, 1999; Antonin et al, 2002). Our data suggest that Ykt6 does not function as a classical SNARE in homotypic vacuole fusion. In fact, Ykt6 addition inhibits liposome–liposome fusion reconstituted with the vacuolar SNAREs Nyv1, Vam3, Vam7 and Vti1 (Fukuda et al, 2000). Moreover, Ykt6 does not function as v-SNARE in liposome-to-liposome fusion, unless its farnesyl anchor, a key feature of this protein, is replaced by a transmembrane domain (McNew et al, 2000). Indeed, we observed that Ykt6 is not found in the trans-SNARE complex (LaGrassa et al, in preparation). Hay and colleagues show that Ykt6 is more stable if purified from the cytosol than from membranes (Hasegawa et al, 2003), suggesting that Ykt6 adopts a closed conformation once released from vacuoles. This conformational switch might regulate Ykt6's acylation activity.
Ykt6 is required at the Golgi (Lupashin et al, 1997; McNew et al, 1997; Tsui and Banfield, 2000; Zhang and Hong, 2002), on late endosomes and on the vacuole (Ungermann et al, 1999; Lewis and Pelham, 2002; Hasegawa et al, 2003; Kweon et al, 2003). It is thus possible that the SNARE domain also targets Ykt6 to fusion complexes to fulfill its role as an acyltransferase. Recently, it was demonstrated that Ykt6 could replace Sec22 in ER to Golgi transport in vivo and in vitro (Liu and Barlowe, 2002). Sec22 and Ykt6 have similarly folded N-termini (Filippini et al, 2001; Gonzalez et al, 2001; Tochio et al, 2001), and thus have the ability to replace each other as SNAREs in the SNARE complex (Liu and Barlowe, 2002), or, potentially, as key players in palmitoylation. The conservation of Ykt6 and Sec18 across species makes it likely that palmitoylation of fusion factors is of general importance. Indeed, where analyzed, a role of Pal-CoA in fusion had been observed (Glick and Rothman, 1987; Pfanner et al, 1990; Haas and Wickner, 1996; Veit et al, 2001).
We have shown that Ykt6 is sufficient to promote in vitro palmitoylation of Vac8 via its N-terminus. This reaction requires roughly an equimolar ratio of Ykt6 and its substrate Vac8 to be efficient. Taking into account the reaction kinetics (Figure 5C,D), we suggest that Ykt6 presents Pal-CoA to Vac8 while transfer under the chosen conditions occurs spontaneously. These conditions favor the target cysteine sulfhydryl group to be reduced and be present as a deprotonated thiolate, which are the requirements for an efficient transfer (Bizzozero et al, 2000). This is in agreement with our analysis using the vacuolar detergent extract as an enzyme source. Modification occurs with similar kinetics, and acylation efficiency depends on the ratio of the added extract to the Vac8-GST substrate (Veit et al, 2003). An alternative explanation would be that Ykt6 alters the conformation of Vac8 to facilitate spontaneous acylation. Such a model does not take into account that Ykt6 binds Pal-CoA and has homology to the β-ketoacyl synthase. Moreover, we did not find any evidence for a complex between Ykt6 and Vac8 in vitro (not shown). We therefore consider this unlikely. Our proposal of a mechanism of directed palmitate transfer would be consistent with all previous reports on putative acyltransferases that transfer palmitate to cysteine residues. Among these are DHHC-CRD proteins that have been introduced as acyltransferases for C-terminal CCaax and CC sequences (Lobo et al, 2002; Roth et al, 2002). Both Erf2/Erf4 and Akr1 have been reconstituted in vitro with their respective substrate. However, in these studies, neither stoichiometry nor kinetics of the transferase reaction were assayed. Interestingly, as in our in vitro assay, similar protein concentrations were used to measure acyltransferases activity in vitro (Lobo et al, 2002; Roth et al, 2002). Importantly, the kinetics of the acylation reaction reported in other studies agree with our observations (Veit et al, 1998; Dunphy et al, 2000). Taking these criteria into account, Ykt6 could also be named an acyltransferase.
During vacuole fusion, Ykt6 has two separable functions. The complete Ykt6 protein is required until tethering, possibly as a chaperone for other SNAREs, a function that we are exploring further. The N-terminal longin domain of Ykt6 acts as the acyltransferase for the fusion factor Vac8 at an early stage of the fusion reaction. This novel step requires Sec18 but not other components necessary for SNARE disassembly, tethering or trans-SNARE pairing. In summary, our data extend the function of SNAREs beyond that of fusion catalysts by demonstrating a role for a SNARE prior to the actual fusion in an essential modification reaction. A single ATPase, NSF/Sec18, controls both pathways.
Materials and methods
Yeast strains and recombinant proteins
The Gal-inducible BJ3505 and DKY6281 strains were generated by chromosomal integration of the Gal promotor in front of the YKT6 open reading frame via PCR (Longtine et al, 1998). Other yeast strains were as described (Haas and Wickner, 1996).
Polyclonal antibodies to Sec17, Sec18, Vam3, Vam2 and Ykt6 were generated as described (Haas and Wickner, 1996; Nichols et al, 1997; Ungermann et al, 1999; Price et al, 2000), and antibodies to the N-terminal 120 amino acids of Ykt6 were generated to a GST fusion protein. Ykt6-wt, Ykt6-R71G, Ykt6N, GST-Vti1, GST-Vti1(1–110), His-Sec17 and His-Sec18 were purified as described (Ungermann et al, 1999; Tochio et al, 2001). Sequencing of His-Ykt6 uncovered a mutation (R71G) within the N-terminus (termed Ykt6-R71G). For the in vitro assay, a wild-type version of His-Ykt6 (Ykt6-wt) was generated. C-terminally tagged Vac8-GST or Ykt6 (1–120)-GST were overproduced from a pETGEX vector in E. coli cells, which, in the case of Vac8-GST, carried a plasmid encoding the human N-myristoyl transferase (Veit et al, 2003).
Vacuole purification and fusion assay
Vacuoles were purified as described (Mayer et al, 1996; Ungermann et al, 1998a). Standard fusion reactions (30 μl) containing 3 μg of each vacuole type were incubated at 26°C in reaction buffer (125 mM KCl, 5 mM MgCl2, 20 mM PIPES/KOH, pH 6.8, 200 mM sorbitol), a protease inhibitor cocktail (Xu and Wickner, 1996), 10 μM CoA, an ATP-regenerating system and cytosol (Haas and Wickner, 1996). One unit of fusion activity is measured as 1 μmol p-nitrophenol phosphate hydrolyzed per min per μg BJ3505 at 30°C.
Palmitoylation assay
[3H]-palmitate labeling of Vac8 on isolated vacuoles was performed as described (Veit et al, 2001). For in vitro acylation, Vac8-GST (2 μM) was incubated in a 100 μl reaction containing reaction buffer (20 mM PIPES/KOH, pH 6.8, 120 mM KCl, 0.015% Triton X-100), His-Ykt6 (2 μM) and [3H]-Pal-CoA (4 μCi; 0.5 μM) for 60 min at 26°C. Proteins were precipitated by the addition of 300 μl of MeOH, 100 μl of chloroform and 400 μl of H2O, vortexed and centrifuged for 5 min at room temperature. The aqueous phase was removed carefully and discarded. Then, 300 μl of MeOH was added, the sample was vortexed and centrifuged again. The protein pellet was dried briefly, resuspended in sample buffer without 2-mercaptoethanol, and analyzed by SDS–PAGE and fluorography.
Acknowledgments
We thank David Banfield for the N-terminal Ykt6 construct, Ed Hurt, Walter Nickel, Felix Wieland and Tracy LaGrassa for critical assessment of the manuscript, and Gabriela Müller for expert technical assistance. This work was supported by a grant from the DFG (UN111/2-3), the EMBO Young Investigator programme, the Fonds der Chemischen Industrie and by a predoctoral fellowship of the BIF (to LEPD).
References
- Antonin W, Fasshauer D, Becker S, Jahn R, Schneider TR (2002) Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat Struct Biol 9: 107–111 [DOI] [PubMed] [Google Scholar]
- Bano MC, Jackson CS, Magee AI (1998) Pseudo-enzymatic S-acylation of a myristoylated yes protein tyrosine kinase peptide in vitro may reflect non-enzymatic S-acylation in vivo. Biochem J 330: 723–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzozero OA, Bixler HA, Pastuszyn A (2001) Structural determinants influencing the reaction of cysteine-containing peptides with palmitoyl-coenzyme A and other thioesters. Biochem Biophys Acta 1545: 278–288 [DOI] [PubMed] [Google Scholar]
- Catchpoole DR, Hong W (1999) Characterization of the sequence and expression of a Ykt6p prenylated SNARE from rat. DNA Cell Biol 18: 141–145 [DOI] [PubMed] [Google Scholar]
- Chen YA, Scheller RH (2001) SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol 2: 98–106 [DOI] [PubMed] [Google Scholar]
- Dilcher M, Kohler B, von Mollard GF (2001) Genetic interactions with the yeast Q-SNARE Vti1 reveal novel functions for the R-SNARE Ykt6. J Biol Chem 276: 34537–34544 [DOI] [PubMed] [Google Scholar]
- Duncan JA, Gilman AG (1996) Autoacylation of G protein alpha subunits. J Biol Chem 271: 23594–23600 [DOI] [PubMed] [Google Scholar]
- Dunphy JT, Schroeder H, Leventis R, Greentree WK, Knudsen JK, Silvius JR, Linder ME (2000) Differential effects of acyl-CoA binding protein on enzymatic and non- enzymatic thioacylation of protein and peptide substrates. Biochim Biophys Acta 1485: 185–198 [DOI] [PubMed] [Google Scholar]
- Faergeman NJ, Knudsen J (1997) Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem J 323: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipini F, Rossi V, Galli T, Budillon A, D'Urso M, D'Esposito M (2001) Longins: a new evolutionary conserved VAMP family sharing a novel SNARE domain. Trends Biochem Sci 26: 407–409 [DOI] [PubMed] [Google Scholar]
- Fukuda R, McNew JA, Weber T, Parlati F, Engel T, Nickel W, Rothman JE, Söllner TH (2000) Functional architecture of an intracellular membrane t-SNARE. Nature 407: 198–202 [DOI] [PubMed] [Google Scholar]
- Glick BS, Rothman JE (1987) Possible role for fatty acyl-coenzyme A in intracellular protein transport. Nature 326: 309–312 [DOI] [PubMed] [Google Scholar]
- Gonzalez LC Jr, Weis WI, Scheller RH (2001) A novel SNARE N-terminal domain revealed by the crystal structure of Sec22b. J Biol Chem 276: 24203–24211 [DOI] [PubMed] [Google Scholar]
- Haas A, Wickner W (1996) Homotypic vacuole fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF). EMBO J 15: 3296–3305 [PMC free article] [PubMed] [Google Scholar]
- Hanley JG, Khatri L, Hanson PI, Ziff EB (2002) NSF ATPase and α-/β-SNAPs disassemble the AMPA receptor–PICK1 complex. Neuron 34: 53–67 [DOI] [PubMed] [Google Scholar]
- Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE (1997) Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell 90: 523–535 [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Zinsser S, Rhee Y, Vik-Moo EO, Davenger S, Hay JC (2003) Mammalian Ykt6 is a neuronal SNARE targeted to a specialized compartment by its profilin-like amino terminal domain. Mol Biol Cell 14: 698–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl TM, Parlati F, Wimmer C, Rothman JE, Söllner TH, Engelhardt H (1998) Arrangement of subunits in 20 S particles consisting of NSF, SNAPs, and SNARE complexes. Mol Cell 2: 539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Lang T, Südhof TC (2003) Membrane fusion. Cell 112: 519–533 [DOI] [PubMed] [Google Scholar]
- Katz L, Hanson PI, Heuser JE, Brennwald P (1998) Genetic and morphological analyses reveal a critical interaction between the C-termini of two SNARE proteins and a parallel four helical arrangement for the exocytic SNARE complex. EMBO J 17: 6200–6209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon Y, Rothe A, Conibear E, Stevens TH (2003) Ykt6p is a multifunctional yeast R-SNARE that is required for multiple membrane transport pathways to the vacuole. Mol Biol Cell 14: 1868–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ, Pelham HRB (2002) A new yeast endosome SNARE related to mammalian syntaxin 8. Traffic 3: 922–929 [DOI] [PubMed] [Google Scholar]
- Liu Y, Barlowe C (2002) Analysis of Sec22p in endoplasmic reticulum/Golgi transport reveals cellular redundancy in SNARE protein function. Mol Biol Cell 13: 3314–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo S, Greentree WK, Linder ME, Deschenes RJ (2002) Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem 277: 41268–41273 [DOI] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961 [DOI] [PubMed] [Google Scholar]
- Lupashin VV, Pokrovskaya ID, McNew JA, Waters MG (1997) Characterization of a novel yeast SNARE protein implicated in Golgi retrograde traffic. Mol Biol Cell 8: 2659–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A (1999) Intracellular membrane fusion: SNAREs only? Curr Opin Cell Biol 11: 447–452 [DOI] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A (1996) Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell 85: 83–94 [DOI] [PubMed] [Google Scholar]
- McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Söllner TH, Rothman JE (2000) Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407: 153–159 [DOI] [PubMed] [Google Scholar]
- McNew JA, Sogaard M, Lampen NM, Machida S, Ye RR, Lacomis L, Tempst P, Rothman JE, Söllner TH (1997) Ykt6p, a prenylated SNARE essential for endoplasmic reticulum–Golgi transport. J Biol Chem 272: 17776–17783 [DOI] [PubMed] [Google Scholar]
- Müller JM, Rabouille C, Newman R, Shorter J, Freemont P, Schiavo G, Warren G, Shima DT (1999) An NSF function distinct from ATPase-dependent SNARE disassembly is essential for Golgi membrane fusion. Nat Cell Biol 1: 335–340 [DOI] [PubMed] [Google Scholar]
- Müller JM, Shorter J, Newman R, Deinhardt K, Sagiv Y, Elazar Z, Warren G, Shima DT (2002) Sequential SNARE disassembly and GATE-16–GOS-28 complex assembly mediated by distinct NSF activities drives Golgi membrane fusion. J Cell Biol 157: 1161–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols BJ, Ungermann C, Pelham HR, Wickner WT, Haas A (1997) Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature 387: 199–202 [DOI] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM (1998) NSF binding to GluR2 regulates synaptic transmission. Neuron 21: 87–97 [DOI] [PubMed] [Google Scholar]
- Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, Ziff EB (1998) The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron 21: 99–110 [DOI] [PubMed] [Google Scholar]
- Pfanner N, Glick BS, Arden SR, Rothman JE (1990) Fatty acylation promotes fusion of transport vesicles with Golgi cisternae. J Cell Biol 110: 955–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier MA, Xiao W, Macosko JC, Chan C, Shin YK, Bennett MK (1998) The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat Struct Biol 5: 765–769 [DOI] [PubMed] [Google Scholar]
- Price A, Seals D, Wickner W, Ungermann C (2000) The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J Cell Biol 148: 1231–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qanbar R, Bouvier M (2003) Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacol Ther 1: 1–33 [DOI] [PubMed] [Google Scholar]
- Rizo J, Südhof TC (2002) SNAREs and munc18 in synaptic vesicle fusion. Nat Rev Neurosci 3: 641–653 [DOI] [PubMed] [Google Scholar]
- Roth AF, Feng Y, Chen L, Davis NG (2002) The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol 159: 23–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE (1994) Mechanisms of intracellular protein transport. Nature 372: 55–63 [DOI] [PubMed] [Google Scholar]
- Sakai T, Ohuchi R, Ohuchi M (2002) Fatty Acids on the A/USSR/77 influenza virus hemagglutinin facilitate the transition from hemifusion to fusion pore formation. J Virol 76: 4603–4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiter R, Guerra CE, Lampl M, Tatzer V, Zellnig G, Klein HL, Kohlwein SD (2000) A novel cold-sensitive allele of the rate-limiting enzyme of fatty acid synthesis, acetyl coenzyme A carboxylase, affects the morphology of the yeast vacuole through acylation of Vac8p. Mol Cell Biol 20: 2984–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Eitzen G, Margolis N, Wickner WT, Price A (2000) A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA 97: 9402–9407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature 362: 318–324 [DOI] [PubMed] [Google Scholar]
- Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir RL (1998) Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron 21: 393–400 [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395: 347–353 [DOI] [PubMed] [Google Scholar]
- Tochio H, Tsui MM, Banfield DK, Zhang M (2001) An autoinhibitory mechanism for nonsyntaxin SNARE proteins revealed by the structure of Ykt6p. Science 293: 698–702 [DOI] [PubMed] [Google Scholar]
- Tsui MM, Banfield DK (2000) Yeast Golgi SNARE interactions are promiscuous. J Cell Sci 113: 145–151 [DOI] [PubMed] [Google Scholar]
- Ungermann C, Nichols BJ, Pelham HR, Wickner W (1998a) A vacuolar v-t-SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J Cell Biol 140: 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Sato K, Wickner W (1998b) Defining the functions of trans-SNARE pairs. Nature 396: 543–548 [DOI] [PubMed] [Google Scholar]
- Ungermann C, von Mollard GF, Jensen ON, Margolis N, Stevens TH, Wickner W (1999) Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J Cell Biol 145: 1435–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Wickner W (1998) Vam7p, a vacuolar SNAP-25 homolog, is required for SNARE complex integrity and vacuole docking and fusion. EMBO J 17: 3269–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M (2000) Palmitoylation of the 25-kDa synaptosomal protein (SNAP-25) in vitro occurs in the absence of an enzyme, but is stimulated by binding to syntaxin. Biochem J 345: 145–151 [PMC free article] [PubMed] [Google Scholar]
- Veit M, Dietrich LEP, Ungermann C (2003) Biochemical characterization of the vacuolar acyltransferase. FEBS Lett 540: 101–105 [DOI] [PubMed] [Google Scholar]
- Veit M, Laage R, Dietrich L, Wang L, Ungermann C (2001) Vac8p release from the SNARE complex and its palmitoylation are coupled and essential for vacuole fusion. EMBO J 20: 3145–3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M, Sachs K, Heckelmann M, Maretzki D, Hofmann KP, Schmidt MFG (1998) Palmitolyation of rhodopsin with S-protein acyltransferase: enzyme catalyzed reaction versus autocatalytic acylation. Biochem Biophys Acta 1394: 90–98 [DOI] [PubMed] [Google Scholar]
- Wang YX, Catlett NL, Weisman LS (1998) Vac8p, a vacuolar protein with armadillo repeats, functions in both vacuole inheritance and protein targeting from the cytoplasm to vacuole. J Cell Biol 140: 1063–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Ungermann C, Wickner W (2000) The docking of primed vacuoles can be reversibly arrested by excess Sec 17p (α-SNAP). J Biol Chem 275: 22862–22867 [DOI] [PubMed] [Google Scholar]
- Wang YX, Kauffman EJ, Duex JE, Weisman LS (2001) Fusion of docked membranes requires the armadillo repeat protein Vac8p. J Biol Chem 276: 35133–35140 [DOI] [PubMed] [Google Scholar]
- Wickner W (2002) Yeast vacuoles and membrane fusion pathways. EMBO J 21: 1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DW, Whiteheart SW, Wiedmann M, Brunner M, Rothman JE (1992) A multisubunit particle implicated in membrane fusion. J Cell Biol 117: 531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski A, Joshi AK, Smith S (2002) Mechanism of the β-ketoacyl synthase reaction catalyzed by the animal fatty acid synthase. Biochemistry 41: 10877–10887 [DOI] [PubMed] [Google Scholar]
- Wurmser AE, Sato TK, Emr SD (2000) New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol 151: 551–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wickner W (1996) Thioredoxin is required for vacuole inheritance in Saccharomyces cerevisiae. J Cell Biol 132: 787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Hong W (2002) Ykt6p forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participates in a late stage in endoplasmic reticulum–Golgi transport. J Biol Chem 276: 27480–27487 [DOI] [PubMed] [Google Scholar]


