Abstract
We previously showed that the energized mitochondrion and extracellular ATP are essential for the candidacidal activity of the N-terminal peptide of human lactoferrin, subsequently referred to as hLF(1-11). The present study focuses on the involvement of internal thiols and reactive oxygen species (ROS) in the candidacidal activity exerted by hLF(1-11). Our results reveal that hLF(1-11) reduced the internal thiol level of Candida albicans by 20%. In agreement, N-acetyl-l-cysteine (NAC), which is a precursor of glutathione and an ROS scavenger, inhibited the candidacidal activity of hLF(1-11). In addition, azodicarboxylic acid bis(N,N-dimethylamide) (diamide), which oxidizes internal thiols, was candidacidal. Furthermore, hLF(1-11) increased the level of ROS production by C. albicans in a dose-dependent manner, and a correlation between ROS production and candidacidal activity was found. 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox), which is an ROS scavenger, partially inhibited the hLF(1-11)-induced, but not the diamide-triggered, candidacidal activity. It is of interest that hLF(1-11) and diamide acted synergistically in killing C. albicans and in ROS production. In agreement, oxidized ATP, an irreversible inhibitor of extracellular ATP receptors, partially blocked the hLF(1-11)-induced, but not the diamide-triggered, candidacidal activity. Finally, the hLF(1-11)-induced activation of mitochondria was inhibited by NAC, indicating that internal thiols and ROS affect mitochondrial activity. Therefore, the candidacidal activity of hLF(1-11) involves both generation of ROS and reduction of internal thiols.
The increasing frequency of Candida albicans systemic infections in immunocompromised patients during recent decades indicates that this yeast is becoming a major human health threat. Amphotericin B, a polyene antimycotic drug discovered in 1956 (11), is the “gold standard” for the treatment of most severe invasive fungal infections, including Candida infections. During the past 15 years a number of new antifungal drugs have been introduced (10), and fluconazole is nowadays the most widely used agent for the treatment of Candida infections (31, 35). Due to intensive prophylactic use of fluconazole in AIDS patients, fluconazole-resistant strains of C. albicans are emerging (17, 33, 41); even strains that are cross resistant to this agent and amphotericin B have been reported (20). In addition, limitations in the spectra of activity, pharmacokinetic properties, and safety of these antimycotic drugs point to the pressing need for new classes of antifungal agents.
Among the different evolving strategies for antifungal therapy (10), those that use antimicrobial proteins and peptides, such as histatin-derived peptides (8, 13, 21) and peptides derived from the N terminus of human lactoferrin (hLF) (24), seem promising. Lactoferrin is a 77-kDa protein present in the specific granules of neutrophils. In response to an inflammatory stimulus, hLF is produced and released by mucosal epithelial cells and neutrophils. This protein exerts antimicrobial activity, which is partly related to its ability to bind iron (5). In addition, hLF releases lactoferricin H, a peptide derived from its N terminus, when it is subjected to pepsinolysis (3). This peptide, which comprises two cationic domains, exhibits more effective antibacterial activity than the native protein (14), and recent studies have indicated that a synthetic peptide representing the first cationic domain, further referred to as hLF(1-11), displays more potent bactericidal and candidacidal activities than the peptide representing the second cationic domain (24, 28). Moreover, the first two N-terminal arginines (residues 2 and 3) are essential for the candidacidal activity of hLF(1-11), as demonstrated by using a peptide in which both arginines were replaced by alanines (24). We recently demonstrated that hLF(1-11) targets energized mitochondria in C. albicans and the level of extracellular ATP (ATPe) has been implicated in hLF(1-11)-induced Candida cell death (24), as has also been described for histatin 5 (12, 13, 21) and human neutrophil defensin 1 (9). It could be inferred that hLF(1-11) interacts with the inner mitochondrial membrane, affecting mitochondrial output (4, 29), e.g., generation of ATP and reactive oxygen species (ROS), as in the drug-induced cytotoxicity in rat hepatocytes (27). Moreover, it has recently been reported that ROS generated by Saccharomyces cerevisiae are involved in the lipid hydroperoxide-induced death of this yeast (2). Since internal thiols, such as glutathione and thioredoxin, protect cells from damage by ROS (32), internal thiol reduction (6, 19, 42) and ROS production have important consequences for cell viability by promoting programmed cell death in both multicellular (15) and unicellular (25) eukaryotes. In view of these data, the present study was undertaken to gain more insight into the involvement of the internal thiols and ROS in the candidacidal activity exerted by the synthetic peptide hLF(1-11).
MATERIALS AND METHODS
Source of C. albicans strain.
Fluconazole-resistant C. albicans strain Y01-19 was purchased from Pfizer Inc. (Groton, Conn.). The yeast was identified by the Candiselect system (Sanofi Pasteur, Paris, France) and was confirmed by demonstration of a typical C. albicans pattern of sugar utilization (API ID 32C; bioMerieux, Marcy l'Etoile, France). Fluconazole resistance (MIC, >256 μg/ml) was evaluated by the E-test (Oxoid Unipath Ltd., Basingstoke, United Kingdom). Yeasts were cultured overnight in Sabouraud broth (Oxoid) at 37°C and were subcultured for 2.5 h on a rotary wheel at 37°C.
Lactoferrin peptides.
The synthetic peptide corresponding to residues 1 to 11 (GRRRRSVQWCA; Mr, 1,494 Da) of hLF [hLF(1-11)] was prepared and purified as described previously (7). Synthetically prepared hLF(1-11) with alanines instead of arginines at positions 2 and 3, further referred to as hLF(1-11)2A/3A, was included as a negative control (24). The purities of both peptides exceeded 88%, as determined by reverse-phase high-performance liquid chromatography. Stocks of peptides at a concentration of 1 mg/ml of 0.01% acetic acid (pH 3.7) were stored at −20°C and were dried in a Speed-Vac vacuum apparatus (Savant Instruments Inc., Farmingdale, N.Y.) immediately before use.
Chemicals.
N-Acetyl-l-cysteine (NAC), azodicarboxylic acid bis(N,N-dimethylamide), further referred to as diamide, and periodate-oxidized ATP (oATP) were purchased from Sigma Chemical Co. (St. Louis, Mo). 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox) was purchased from Calbiochem-Novabiochem Corp. (La Jolla, Calif.). Stocks of diamide (1 M) and oATP (100 mM) were prepared in phosphate-buffered saline (pH 7.4) and were stored at −20°C until use. A stock of trolox (100 mM) was prepared in methanol and was stored at −20°C until use. NAC was freshly prepared in 10 mM of sodium phosphate buffer (NaPB; pH 7.4).
Treatment of C. albicans with NAC, diamide, trolox, or oATP.
C. albicans cells were preincubated with trolox (5 mM) or oATP (0.3 mM) for 30 min at 37°C or with various concentrations of diamide for 10 min at 37°C prior to addition of the stimulus. NAC (20 mM) was added simultaneously with the stimulus to C. albicans cells.
Assay for candidacidal activity of hLF(1-11).
An in vitro assay (24) was used to assess the candidacidal activity of hLF(1-11). Briefly, yeast cells were harvested in the mid-log phase by centrifugation (1,500 × g, 10 min), washed twice in NaPB, and diluted to a concentration of 106 CFU/ml of NaPB supplemented with 2% (vol/vol) Sabouraud broth. Equal volumes of this suspension and various concentrations of hLF(1-11) were mixed in Eppendorf vials. After incubation for 2 h at 37°C with hLF(1-11), the vials were transferred to ice and the number of viable blastoconidia was determined by plating serial dilutions of each sample on Sabouraud agar. Results are expressed as the number of CFU of C. albicans per milliliter. Preliminary experiments indicated that the candidacidal activity of hLF(1-11) was stopped at 4°C.
Measurement of ROS production.
2′,7′-Dichlorofluorescein diacetate (DCFH-DA; Eastman Kodak Company, Rochester, N.Y.) was used to measure the level of ROS production by Candida. C. albicans cells were harvested in the mid-log phase, washed twice as described above, and then diluted to a concentration of 2 × 106 CFU/ml of NaPB. Next, the Candida cells were preincubated for 15 min at 37°C with 100 μM DCFH-DA and were then treated for 15 min at 37°C with various concentrations of hLF(1-11). Immediately before use, DCFH-DA (100 mM) was dissolved in dimethyl sulfoxide and was further diluted in NaPB. The fluorescence of DCF was measured on a fluorescence-activated cell sorter (FACS; FACScan; Becton Dickinson and Co., San Jose, Calif.). Results are expressed as median fluorescence intensities.
Measurement of intracellular thiol levels.
A stock (100 mM) of monochlorobimane (MCB; Molecular Probes, Eugene, Oreg.) was prepared in dimethyl sulfoxide and was stored at 4°C until use. To measure the levels of intracellular thiols, the cells were incubated with MCB, a membrane-permeant nonfluorescent compound that becomes fluorescent after reaction with sulfhydryl groups (34), by the method described by Staal et al. (38), with minor modifications. In short, mid-log-phase C. albicans cells were diluted to a concentration of 2 × 106 CFU/ml of NaPB. Next, the cells were incubated for 30 min at 37°C with various concentrations of hLF(1-11). The reaction was stopped by transferring the tubes onto ice for 5 min and then exposing the cells for 20 min at 4°C to MCB at a final concentration of 40 μM. The amount of free sulfhydryl groups in the cells was analyzed by flow cytometry. The results are expressed as the median fluorescence intensities.
Assay for mitochondrial activity.
A stock (100 mM) of the fluorescent probe rhodamine 123 (Molecular Probes) was prepared in methanol and stored at 4°C until use. The mitochondrial activity of C. albicans was investigated by using rhodamine 123 (24), which is a positively charged probe that accumulates in mitochondria, depending on the mitochondrial transmembrane potential (18). Briefly, C. albicans cells in the mid-log phase were washed in potassium phosphate buffer (PPB; 1 mM [pH 7.0]) and were resuspended at a concentration of 2 × 106 CFU/ml in PPB. They were then incubated for 10 min at 37°C with 10 μM rhodamine 123 in PPB. After washes with PPB, the cells were mixed with 17 μM hLF(1-11) and were immediately prepared for microscopic inspection of the distribution of rhodamine 123 fluorescence with a fluorescent microscope (Axiolab; Zeiss, Württenburg, Germany).
ATP bioluminescence assay.
ATP levels in cultures of C. albicans were measured as described previously (24). Briefly, mid-log-phase yeast cells were diluted to a concentration of 108 CFU/ml of NaPB. Equal volumes of this suspension and various concentrations of hLF(1-11) were mixed. After incubation at 37°C for various intervals the reaction was stopped by transferring the tubes onto ice for 5 min and then centrifuging them (10,000 × g, 10 min) at 4°C. The supernatants were collected, and the cells were resuspended in an equal volume of phosphate-buffered saline. The cell suspensions were boiled for an additional 3 min. Extracellular and intracellular ATP levels were measured by luminometry with an ATP determination kit (Molecular Probes), according to the instructions of the manufacturer. Briefly, a luciferin-luciferase assay mixture (180 μl) was added to 20 μl of cell lysates or supernatants; 150 μl of each sample was transferred to a 96-well microtiter plate, and light emission was monitored with a 1420 Multilabel Counter-Wallac Victor 2 luminometer (EG&G Wallac, Turku, Finland). The light emitted from the samples was measured as bioluminescence relative light units, and ATP concentrations were calculated by using a standard curve constructed for various concentrations of ATP.
Statistical analysis.
The results are presented as the means plus standard deviations of at least three independent experiments. Differences between the values were analyzed by the Mann-Whitney U test. The correlation between candidacidal activity and ROS production induced upon the addition of hLF(1-11) was analyzed by the Spearman rank test. The level of significance was set at a P value of <0.05.
RESULTS
Effect of hLF(1-11) on production of ROS by C. albicans.
Since ROS may be implicated in the candidacidal activity of hLF(1-11), the production of ROS by C. albicans upon exposure to this peptide was measured. FACS analysis demonstrated that within the first 15 min hLF(1-11) increased (P < 0.05) the level of cellular ROS in C. albicans in a dose-dependent manner (Fig. 1). In agreement, in the presence of high concentrations (67 μM) of the peptide hLF(1-11)2A/3A, which was not candidacidal (24), no ROS production could be detected in C. albicans (Fig. 1).
FIG. 1.
Effects of NAC, diamide, and trolox on the dose-dependent killing of fluconazole-resistant C. albicans by hLF-derived peptide hLF(1-11). Cells were incubated with various concentrations of hLF(1-11) in the presence of 20 mM NAC (hatched bars), 5 mM diamide (vertically hatched bars), or 5 mM trolox (closed bars) or were left untreated (dotted bars). After incubation at 37°C for 2 h, the number of viable Candida cells was determined microbiologically. The results are the means plus standard deviations of at least three independent experiments. In addition, the effects of these compounds on hLF(1-11)-triggered ROS production by C. albicans were assessed by FACS analysis. Cells were preincubated for 15 min at 37°C with 100 μM DCFH-DA and were then treated for 15 min at 37°C with various concentrations of hLF(1-11) in the presence of 20 mM NAC (hatched bars), 2 mM diamide (vertically hatched bars), or 5 mM of trolox (closed bars) or were left untreated (dotted bars). The results are expressed as the median fluorescence intensities, and the values are the means plus standard deviations of at least three independent experiments. no, no peptide. The peptide in which the first and second arginines were replaced by alanines, hLF(1-11)2A/3A (open bars), was used as the negative control.
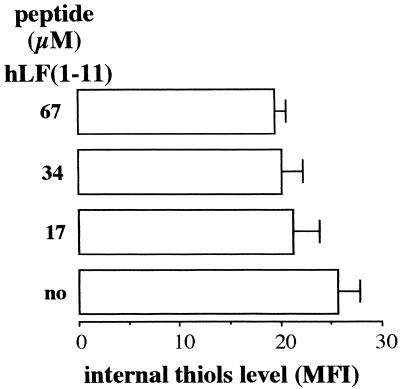
Effect of hLF(1-11) on levels of thiols in C. albicans.
Since internal thiols, the major one being glutathione (γ-glutamylcysteinylglycine [GSH]), buffer the toxic effects of ROS in cells (26), the effect of hLF(1-11) on the levels of thiols in Candida was investigated. FACS analysis revealed a 20% ± 3% decrease (P < 0.05; n = 5) in intracellular thiol levels upon exposure to the hLF(1-11) peptide (Fig. 2).
FIG. 2.
Effect of hLF(1-11) on the levels of internal thiols. Cells were incubated for 30 min at 37°C with various concentrations of hLF(1-11). The reaction was stopped by transferring the vials to ice, and then the cells were incubated for 20 min at 4°C with 40 μM MCB. The amount of free sulfhydryl groups in the cells was analyzed by flow cytometry. no, no peptide. The results are expressed as median fluorescence intensities, and the values are the means plus standard deviations of at least three independent experiments.
Effect of NAC on hLF(1-11)-induced candidacidal activity and ROS production.
Next, the effect of NAC, which is a precursor of GSH and a scavenger for ROS, on hLF(1-11)-induced candidacidal activity was determined. The results revealed that 20 mM NAC rescued (P < 0.05) the cells from the candidacidal activity of this peptide (Fig. 1). NAC at this concentration did not affect the viability of C. albicans. In addition, NAC blocked ROS production (P < 0.05) by Candida upon exposure to hLF(1-11) (Fig. 1).
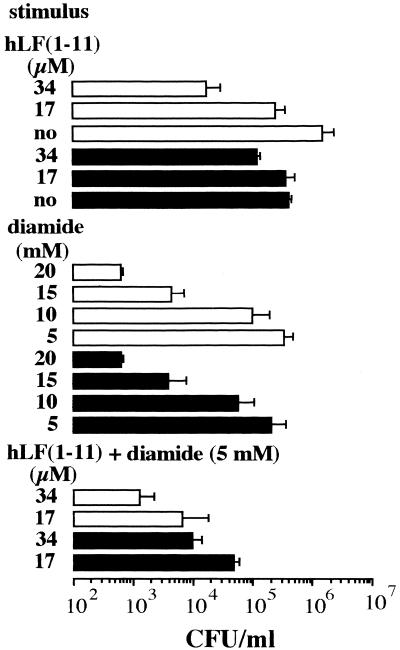
Effects of diamide on viability of and ROS production by C. albicans.
In order to investigate whether reduction of the levels of internal thiols is sufficient to kill Candida, the effects of various concentrations (5 to 20 mM) of diamide, which nonenzymatically converts GSH to the oxidized dimer glutamylcysteine GS-SG (22), on Candida viability was measured. The results revealed that diamide displays a candidacidal effect in a dose-dependent manner (Fig. 3). The candidacidal activity of diamide was almost completely inhibited (P < 0.05) by NAC, indicating that its toxic effect can be attributed to its effect on internal thiols. Diamide did not induce ROS production by Candida (Fig. 1).
FIG. 3.
Effects of oATP on the candidacidal activity of hLF(1-11), diamide, and the combination of diamide and hLF(1-11). Cells were preincubated in the presence of 0.3 mM oATP (closed bars) or without this agent (open bars) for 30 min before addition of various concentrations of hLF(1-11), diamide, or the combination of noncandidacidal concentrations of these agents. After incubation at 37°C for 2 h, the number of viable Candida cells was determined microbiologically. no, no peptide. The results are the means plus standard deviations of at least three independent experiments.
To further investigate the role of free sulfhydryl groups on the candidacidal activity of hLF(1-11), the effect of a noncandidacidal concentration of diamide (5 mM) in combination with suboptimal concentrations of hLF(1-11) on the viability of Candida was determined. The results revealed that hLF(1-11) had significantly (P < 0.05) increased candidacidal activity in the presence of diamide (Fig. 1), indicating synergism between these compounds. It is of interest that this combination of diamide (2 mM) and hLF(1-11) induced an increase in the level of ROS production by C. albicans (Fig. 1).
Relationship between ROS production and candidacidal activity of hLF(1-11).
To further investigate the role of ROS in the candidacidal activity of hLF(1-11), the effect of trolox, a scavenger of ROS, on the candidacidal activity of hLF(1-11) was measured. The results revealed that the candidacidal activity induced by 67 μM hLF(1-11), but not lower concentrations of this peptide, was significantly (P < 0.05) inhibited by trolox (Fig. 1). Furthermore, trolox did not inhibit the diamide-induced candidacidal activity (data not shown). In addition, a significant (P < 0.001; r = −0.798) correlation was found between candidacidal activity and the level of ROS production.
Effect of oATP on candidacidal activities of hLF(1-11), diamide, and the combination of noncandidacidal concentrations of these agents.
Since preincubation with oATP, which irreversibly blocks the interaction between ATPe and its receptors (23), is known to inhibit the candidacidal activity of hLF(1-11) (24), we investigated the effect of oATP on the killing of C. albicans upon exposure to diamide and the combination of hLF(1-11) and diamide. No effect of oATP on the candidacidal activity of optimal concentrations of diamide was observed (Fig. 3), indicating that this activity was not dependent on ATPe. The protective effect (P < 0.05) of oATP against the candidacidal effect of the combination of hLF(1-11) and diamide amounted to 1 log (Fig. 3), which was similar to the effect of oATP on the hLF(1-11)-induced candidacidal activity. Our observation that oATP significantly (P < 0.05) inhibited hLF(1-11)-induced ROS production indicates that ATPe is essential for ROS production.
Effects of NAC and diamide on hLF(1-11)-stimulated mitochondrial activity and increase in ATPe level.
Next, the involvement of intracellular thiols and ROS production in the energizing effect of hLF(1-11) on the mitochondria of C. albicans was investigated by using C. albicans cells preloaded with the fluorescent probe rhodamine 123 (24). The results revealed that NAC blocked the energizing activity of hLF(1-11) in virtually all C. albicans cells, as indicated by a lack of mitochondrial staining (data not shown). In addition, diamide in combination with hLF(1-11) energized mitochondria to a lesser extent than hLF(1-11) alone. In agreement with these observations, we found that the hLF(1-11)-induced increase (P < 0.05) in the ATPe level was completely blocked by NAC and was significantly (P < 0.05) reduced by diamide (Fig. 4). In addition, diamide itself did not affect the ATPe level.
FIG. 4.
Effects of NAC and diamide on the hLF(1-11)-induced increase in ATPe levels. C. albicans cells were incubated with 8 μM hLF(1-11) (closed symbols) or without the peptide (open symbols). Cells were either preincubated with 8 mM diamide (triangles) or incubated with 20 mM NAC (circles) or without these compounds (squares). The ATPe level was measured at various intervals with an ATP determination kit. The results are expressed as the means plus standard deviations of at least three independent experiments.
DISCUSSION
The main conclusion from the present results is that reduced internal thiols and the production of ROS are involved in the candidacidal activity of hLF(1-11). This conclusion is based on the following findings. First, a 20% reduction in the levels of internal thiols after addition of hLF(1-11) was found. NAC, which increases the levels of internal thiols, significantly decreased the hLF(1-11)-induced candidacidal activity. Diamide, which rapidly consumes intracellular GSH by nonenzymatically converting it to the dimer GS-SG (22), was candidacidal, and NAC blocked this activity completely. Second, it is known that because of its antioxidant properties, GSH, which constitutes the largest component of the internal thiol buffer (6, 36), protects cells from oxidative damage. Indeed, hLF(1-11) induced ROS production by C. albicans in a dose-dependent manner, and a significant correlation was found between the hLF(1-11)-induced level of ROS and its candidacidal activity. Trolox, which scavenges ROS, reduced the level of hLF(1-11)-induced candidacidal activity. In agreement, in the presence of hLF(1-11)2A/3A, which showed no candidacidal activity, no ROS production could be detected in C. albicans.
Another important conclusion pertains to the roles of internal thiols in the hLF(1-11)-induced energization of mitochondria and in the increased level of ATPe. NAC blocked the hLF(1-11)-induced increase in ATPe levels and inhibited the energizing effect of hLF(1-11) on mitochondria, indicating that ROS and internal thiols are essential for the stimulation of mitochondrial activities by hLF(1-11). Diamide, which did not influence mitochondria or the ATPe level, reduced the level of hLF(1-11)-induced energization of mitochondria as well as increased the ATPe level, suggesting that it deenergizes mitochondria. In agreement with data published by Zorov et al. (44), these deenergized mitochondria may produce significantly elevated levels of ROS. In this connection, it is of interest that diamide, which at a noncandidacidal concentration did not induce a significant increase in the level of ROS, significantly increased the level of hLF(1-11)-induced ROS production by C. albicans and the candidacidal activity. Furthermore, an irreversible inhibitor of ATPe receptors, oATP, which inhibits the hLF(1-11)-induced candidacidal activity (24), was partly able to inhibit the hLF(1-11)-induced ROS production, suggesting that ROS production is partly ATPe dependent. The candidacidal activity of diamide was neither inhibited by oATP nor increased by the addition of the ATP analogue benzoyl-benzoyl-ATP (data not shown).
Another finding of this investigation is that oATP induced a 1-log reduction in the candidacidal activity exerted by hLF(1-11) alone or the combination of hLF(1-11) and diamide. These data together suggest an alternative candidacidal mechanism of diamide, perhaps through reduction of internal thiols. As expected, the candidacidal effect of diamide was not inhibited by trolox.
The present and previous data (24) together indicate that the candidacidal activity exerted by hLF(1-11) involves the energized mitochondrion, reduction of internal thiols, and production of ROS and ATP. The results of experiments with inhibitors and/or activators of these biochemical changes in Candida underscore their possible role in the candidacidal activity of this peptide (Fig. 5). If we assume that such a relationship exists, the main question that remains is how the ROS and internal thiols are involved in the candidacidal activity of hLF(1-11). It could well be that they mediate some form of programmed cell death, as described for aging S. cerevisiae cells (37) or after expose of this yeast to hydrogen peroxide (25). It has been shown that the reduction-oxidation (redox) state of the cell, which is a consequence of the balance between the internal thiol and ROS levels, modulates the DNA-binding affinities of several mammalian transcription factors, such as c-Fos and c-Jun (1), NF-κB (39), p53 (40), and c-Myb (26). However, studies with anucleate cells (16) and cell-free systems consisting of purified mitochondria indicate that a soluble factor(s) released from mitochondria undergoing changes in permeability induces chromatin condensation in isolated nuclei (43). Since programmed cell death is a common cell behavior, it would be interesting to clarify whether the candidacidal activity of hLF(1-11) is mediated by an effect on the binding affinity of DNA transcription factors, as described for yeasts (30), or by the release of a soluble factor(s) from mitochondria into the cytoplasm of C. albicans.
FIG. 5.
Simplified representation of the mechanisms underlying the candidacidal activity of hLF(1-11). The peptide interacts with its binding sites in the cell envelope of Candida and is taken up by this yeast in an energy-dependent fashion. Subsequently, the peptide interacts with energized mitochondria, causing the production of a number of factors, including ATP and ROS. The latter may lead to a reduction in internal thiol levels, whereas ATP interacts with extracellular-specific binding sites in the cell envelope. These events mediate cellular processes that lead to cell death.
Acknowledgments
This work was supported by a research grant from the Italian “Ministero dell’ Università e della Ricerca Scientifica e Tecnologica,” contract no. MM06248147 and 2001064775.
REFERENCES
- 1.Abate, C., L. Patel, F. J. Rauscher, and T. Curran. 1990. Redox regulation of Fos and Jun DNA-binding activity in vitro. Science 249:1157-1161. [DOI] [PubMed] [Google Scholar]
- 2.Aoshima, H., K. Kadoya, H. Taniguchi, T. Satoh, and H. Hatanaka. 1999. Generation of free radicals during the death of Saccharomyces cerevisiae caused by lipid hydroperoxide. Biosci. Biotechnol. Biochem. 63:1025-1031. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy, W., H. Wakabayashi, M. Takase, K. Kawase, S. Shimamura, and M. Tomita. 1993. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med. Microbiol. Immunol. 182:97-105. [DOI] [PubMed] [Google Scholar]
- 4.Biswal, S. S., K. Datta, S. D. Shaw, X. Feng, J. D. Robertson, and J. P. Kehrer. 2000. Glutathione oxidation and mitochondrial depolarization as mechanisms of nordihydroguaiaretic acid-induced apoptosis in lipoxygenase-deficient FL5.12 cells. Toxicol. Sci. 53:77-83. [DOI] [PubMed] [Google Scholar]
- 5.Bullen, J. J. 1981. The significance of iron in infection. Rev. Infect. Dis. 3:1127-1138. [DOI] [PubMed] [Google Scholar]
- 6.Davis, W., Jr., Z. Ronai, and K. D. Tew. 2001. Cellular thiols and reactive oxygen species in drug-induced apoptosis. J. Pharmacol. Exp. Ther. 296:1-6. [PubMed] [Google Scholar]
- 7.de Koster, H. S., R. Amons, W. E. Benckhuijsen, M. Feijlbrief, G. A. Schellekens, and J. W. Drijfhout. 1995. The use of dedicated peptide libraries permits the discovery of high affinity binding peptides. J. Immunol. Methods 187:179-188. [DOI] [PubMed] [Google Scholar]
- 8.Edgerton, M., S. E. Koshlukova, T. E. Lo, B. G. Chrzan, R. M. Straubinger, and P. A. Raj. 1998. Candidacidal activity of salivary histatins. Identification of a histatin 5-binding protein on Candida albicans. J. Biol. Chem. 273:20438-20447. [DOI] [PubMed] [Google Scholar]
- 9.Edgerton, M., S. E. Koshlukova, M. W. B. Araujo, R. C. Patel, J. Dong, and J. A. Bruenn. 2000. Salivary histatin 5 and human neutrophil defensin 1 kill Candida albicans via shared pathways. Antimicrob. Agents Chemother. 44:3310-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgopapadakou, N. H., and T. J. Walsh. 1996. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother. 40:279-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold, W., H. A. Stout, J. F. Pagano, and R. Donovick. 1955-1956. Amphotericins A and B, antifungal antibiotics produced by a Streptomycete. I. In vitro studies, p. 579-586. Antibiot. Ann. [PubMed]
- 12.Gyurko, C., U. Lendenmann, R. F. Troxler, and F. G. Oppenheim. 2000. Candida albicans mutants deficient in respiration are resistant to the small cationic salivary antimicrobial peptide histatin 5. Antimicrob. Agents Chemother. 44:348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helmerhorst, E. J., P. Breeuwer, W. van't Hof, E. Walgreen-Weterings, L. C. J. M. Oomen, E. C. I. Veerman, A. V. Nieuw Amerongen, and T. Abee. 1999. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 274:7286-7291. [DOI] [PubMed] [Google Scholar]
- 14.Hoek, K. S., J. M. Milne, P. A. Grieve, D. A. Dionysius, and R. Smith. 1997. Antibacterial activity in bovine lactoferrin-derived peptides. Antimicrob. Agents Chemother. 41:54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hug, H., S. Strand, A. Grambihler, J. Galle, V. Hack, W. Stremmel, P. H. Krammer, and P. R. Galle. 1997. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J. Biol. Chem. 272:28191-28193. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson, M. D., J. F. Burne, and M. C. Raff. 1994. Programmed cell death and Bcl-2 protection in the absence of a nucleus. EMBO J. 13:1899-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, E. M., D. W. Warnock, J. Luker, S. R. Porter, and C. Scully. 1995. Emergence of azole drug resistance in Candida species from HIV-infected patients receiving prolonged fluconazole therapy for oral candidosis. J. Antimicrob. Chemother. 35:103-114. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, L. V., M. L. Walsh, and L. B. Chen. 1980. Localization of mitochondria in living cells with rhodamine 123. Proc. Natl. Acad. Sci. USA 77:990-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannan, K., and S. K. Jain. 2000. Oxidative stress and apoptosis. Pathophysiology 7:153-163. [DOI] [PubMed] [Google Scholar]
- 20.Kelly, S. L., D. C. Lamb, D. E. Kelly, J. Loeffler, and H. Einsele. 1996. Resistance to fluconazole and amphotericin in Candida albicans from AIDS patients. Lancet 348:1523-1524. [DOI] [PubMed] [Google Scholar]
- 21.Koshlukova, S. E., M. W. B. Araujo, D. Baev, and M. Edgerton. 2000. Released ATP is an extracellular cytotoxic mediator in salivary histatin 5-induced killing of Candida albicans. Infect. Immun. 68:6848-6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosower, E. M., and N. S. Kosower. 1969. Lest I forget thee, glutathione. Nature 224:117-120. [DOI] [PubMed] [Google Scholar]
- 23.Lammas, D. A., C. Stober, C. J. Harvey, N. Kendrick, S. Panchalingam, and D. S. Kumararatne. 1997. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity 7:433-444. [DOI] [PubMed] [Google Scholar]
- 24.Lupetti, A., A. Paulusma-Annema, M. M. Welling, S. Senesi, J. T. van Dissel, and P. H. Nibbering. 2000. Candidacidal activities of human lactoferrin peptides derived from the N terminus. Antimicrob. Agents Chemother. 44:3257-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madeo, F., E. Fröhlich, M. Ligr, M. Grey, S. J. Sigrist, D. H. Wolf, and K.-U. Fröhlich. 1999. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 145:757-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myrset, A. H., A. Bostad, N. Jamin, P. N. Lirsac, F. Toma, and O. S. Gabrielsen. 1993. DNA and redox state induced conformational changes in the DNA-binding domain of the Myb oncoprotein. EMBO J. 12:4625-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa, Y., and G. Moore. 1999. Role of mitochondrial membrane permeability transition in p-hydroxybenzoate ester-induced cytotoxicity in rat hepatocytes. Biochem. Pharmacol. 58:811-816. [DOI] [PubMed] [Google Scholar]
- 28.Nibbering, P. H., E. Ravensbergen, M. M. Welling, L. A. van Berkel, P. H. C. van Berkel, E. K. J. Pauwels, and J. H. Nuijens. 2001. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect. Immun. 69:1469-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nieminen, A.-L., A. K. Saylor, S. A. Tesfai, B. Herman, and J. J. Lemasters. 1995. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem. J. 307:99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinson, B., O. S. Gabrielsen, and B. Daignan-Fornier. 2000. Redox regulation of AMP synthesis in yeast: a role of the Bas1p and Bas2p transcription factors. Mol. Microbiol. 36:1460-1469. [DOI] [PubMed] [Google Scholar]
- 31.Powderly, W. G. 1994. Resistant candidiasis. AIDS Res. Hum. Retrovir. 10:925-929. [DOI] [PubMed] [Google Scholar]
- 32.Powis, G., D. L. Kirkpatrick, M. Angulo, and A. Baker. 1998. Thioredoxin redox control of cell growth and death and the effects of inhibitors. Chem. Biol. Interact. 111-112:23-34. [DOI] [PubMed] [Google Scholar]
- 33.Rex, J. H., M. G. Rinaldi, and M. A. Pfaller. 1995. Resistance of Candida species to fluconazole. Antimicrob. Agents Chemother. 39:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice, G. C., E. A. Bump, D. C. Shrieve, W. Lee, and M. Kovacs. 1986. Quantitative analysis of cellular glutathione by flow cytometry utilizing monochlorobimane: some applications to radiation and drug resistance in vitro and in vivo. Cancer Res. 46:6105-6110. [PubMed] [Google Scholar]
- 35.Sanglard, D., F. Ischer, M. Monod, and J. Bille. 1996. Susceptibilities of C. albicans multidrug transporter mutants to various antifungal agents and other metabolic inhibitors. Antimicrob. Agents Chemother. 40:2300-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schroder, C. P., A. K. Godwin, P. J. O'Dwyer, K. D. Tew, T. C. Hamilton, and R. F. Ozols. 1996. Glutathione and drug resistance. Cancer Investig. 14:158-168. [DOI] [PubMed] [Google Scholar]
- 37.Sinclair, D. A., K. Mills, and L. Guarente. 1998. Molecular mechanisms of yeast aging. Trends Biochem. Sci. 23:131-134. [DOI] [PubMed] [Google Scholar]
- 38.Staal, F. J. T., M. Roederer, L. A. Herzenberg, and L. A. Herzenberg. 1990. Intracellular thiols regulate activation of nuclear factor κB and transcription of human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 87:9943-9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toledano, M. B., and W. J. Leonard. 1991. Modulation of transcription factor NF-κB binding activity by oxidation-reduction in vitro. Proc. Natl. Acad. Sci. USA 88:4328-4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhaegh, G. W., M. J. Richard, and P. Hainaut. 1997. Regulation of p53 by metal ions and by antioxidants: dithiocarbamate down-regulates p53 DNA-binding activity by increasing the intracellular level of copper. Mol. Cell. Biol. 17:5699-5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vuffray, A., C. Durussel, P. Boerlin, F. Boerlin-Petzold, J. Bille, M. P. Glauser, and J. P. Chave. 1994. Oropharyngeal candidiasis resistant to single-dose therapy with fluconazole in HIV-infected patients. AIDS 8:708-709. [DOI] [PubMed] [Google Scholar]
- 42.Yoo, Y.-C., R. Watanabe, Y. Koike, M. Mitobe, K.-I. Shimazaki, S. Watanabe, and I. Azuma. 1997. Apoptosis in human leukemic cells induced by lactoferricin, a bovine milk protein-derived peptide: involvement of reactive oxygen species. Biochem. Biophys. Res. Commun. 237:624-628. [DOI] [PubMed] [Google Scholar]
- 43.Zamzami, N., S. A. Susin, P. Marchetti, T. Hirsch, I. Gómez-Monterrey, M. Castedo, and G. Kroemer. 1996. Mitochondrial control of nuclear apoptosis. J. Exp. Med. 183:1533-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zorov, D. B., C. R. Filburn, L. O. Klotz, J. L. Zweier, and S. J. Sollott. 2000. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 192:1001-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]