Abstract
Insects produce several types of peptides to combat a broad spectrum of invasive pathogenic microbes, including protozoans. However, despite this defense response, infections are often established. Our aim was to design novel peptides that produce high rates of mortality among protozoa of the genus Plasmodium, the malaria parasites. Using existing antimicrobial peptide sequences as templates, we designed and synthesized three short novel hybrids, designated Vida1 to Vida3. Each has a slightly different predicted secondary structure. The peptides were tested against sporogonic stages of the rodent malaria parasites Plasmodium berghei (in vitro and in vivo) and P. yoelii nigeriensis (in vitro). The level of activity varied for each peptide and according to the parasite stage targeted. Vida3 (which is predicted to have large numbers of β sheets and coils but no α helices) showed the highest level of activity, killing the early sporogonic stages in culture and causing highly significant reductions in the prevalence and intensity of infection of P. berghei after oral administration or injection in Anopheles gambiae mosquitoes. The secondary structures of these peptides may play a crucial role in their ability to interact with and kill sporogonic forms of the malaria parasite.
Most naturally occurring antimicrobial peptides have broad spectra of activity and are believed to permeabilize the lipid bilayer of target microbes (8). These molecules are one of the main components of the innate immune response of insects (7), and considerable research effort has been directed toward understanding their activities against disease-causing pathogens and parasites transmitted by insect vectors.
Mosquitoes are vectors for many of the major tropical diseases and, as such, are the focus for much of this work. Upon invasion or injury, the immune system is activated, expressing several molecules, including antimicrobial peptides (21, 23, 25). Two classes of peptides, defensins and cecropins, and a novel peptide, gambicin, have been identified in the Anopheles gambiae mosquito vector of malaria (28, 40, 41).
Following male gametocyte exflagellation within the malaria parasite-infected blood meal, gametes fuse to form zygotes, which develop within the midgut lumen into extracellular, motile ookinetes (>15 h after the blood meal). The ookinete traverses the midgut, forming an oocyst beneath the basal lamina (24 to 30 h). Following several days of development, the oocysts release numerous infective sporozoites (12 to 15 days), which travel to the salivary glands, where they reside prior to their transmission the next time the mosquito takes a blood meal (34). All sporogonic stages of the rodent malaria parasite, Plasmodium berghei, can now be grown in vitro (3).
Immune activation both prior to and immediately after parasite ingestion has been shown to reduce the levels of malaria parasite infection within the mosquito vector (22). Genes encoding defense molecules have been shown experimentally to be upregulated by malaria parasite infection (29). However, any upregulation of mRNA defense molecules that may occur following an infectious blood meal does not result in the death of all the malaria parasites in competent mosquitoes.
Attempts to target sporogonic stages in vitro, as well as to reduce the level of malaria parasite infection within the mosquito, have produced mixed results. Injection or feeding of natural peptides such as cecropins and magainins (14) and defensins (31) or hybrid peptides such as the cecropin-like peptides Shiva-3 (27) and scorpine (11) into infected mosquitoes reduced parasite levels significantly. However, most of the peptides had to be used at very high concentrations to induce any significant effect and often were active against only some sporogonic stages of development (14, 27, 31). In the case of scorpine, it was shown to be very active at inhibiting gametogenesis and ookinete formation at low concentrations both in vitro and in vivo, but it has not been tested against the later stages, such as oocysts and sporozoites (11). Finally, these peptides are between 29 and 75 amino acids in length, making them difficult and expensive to synthesize.
Most naturally occurring antimicrobial peptides have a size range ≥20 amino acids, which is a sufficient length to span and depolarize the lipid bilayer. Shorter and longer active molecules can also be found. Hybrids have been designed which incorporate specific regions, motifs, or sequences from naturally occurring peptides (16) that are thought to be important in the formation of an active peptide.
Previously, short hybrid peptides have been created which are less than 20 amino acids in length, i.e., below the minimum length believed to be required to span a lipid bilayer (4). These peptides often have MICs lower than those of the larger hybrids and naturally occurring peptides, while they retain their broad spectra of activity (4, 12, 36). This suggests that, compared to the larger peptides, short peptides may use a different mechanism to interact with or span target membranes to induce their mode of action.
Hybrid and naturally occurring peptides share two very important properties which are believed to be essential in the creation of an active antimicrobial peptide. These are amphipathicity (the distribution of a positive or a negative charge that spans the entire peptide) and flexibility (which allows insertion or incorporation of the peptide into the lipid bilayer). Ultimately, the variabilities in amino acid contents, sizes, and secondary structures are likely to lead to differences in activity between each class or type of antimicrobial peptide against different types of microorganisms (8).
The specific aim of this project was to produce novel peptides that are highly active against protozoa. Using both natural and synthetic hybrids as templates, we designed three short hybrid peptides that show a range of predicted secondary structures. The novel peptides were tested against two species of rodent malaria parasites. Both in vivo and in vitro experiments showed that the predicted secondary structure plays a crucial role in the level of peptide activity against Plasmodium spp. Those peptides with a predicted secondary structure that consists mainly of random coils and turns (i.e., no specific characteristics in the secondary structure) are particularly active against the sporogonic stages of P. berghei and P. yoelii nigeriensis.
MATERIALS AND METHODS
Mosquito maintenance.
A. gambiae strain KIL was maintained at 26 ± 1°C and in a 12-h light, 12-h dark cycle. Stock larvae were reared under standardized conditions (17). Adults were fed a mixture of 10% glucose with antibiotics (14 U of penicillin per ml, 14 μg of streptomycin per ml in 0.9% sodium chloride) and 0.05% p-aminobenzoic acid ad libitum (26).
Parasite maintenance and mosquito infection.
P. berghei strain ANKA, clone 2.34, was maintained in male CD mice through no more than eight mechanical blood passages (33) with regular transmission through mosquitoes. P. yoelii nigeriensis Killick-Kendrick (strain N67) was also maintained in male CD mice, but through only up to two blood passages. Levels of parasitemia and gametocytemia were monitored by using stained, thin blood smears and fresh, thick blood smears. Mosquitoes infected with P. berghei were then maintained at 20 ± 1°C, as this is the optimum temperature for P. berghei sporogonic development. Mosquitoes infected with P. yoelii nigeriensis were maintained at 26°C.
Peptide design.
Following an extensive survey of the literature, the conclusion was drawn that certain amino acids, sequences, motifs, and arrangements from both natural and hybrid peptides were more likely to be effective against complex outer membranes, such as those from fungi or protozoa. This pattern appeared to be related to a difference in peptide secondary structure (a helical structure for bacteria and more random structures for fungi and protozoa). The computer program Protean (part of the Lasergene molecular biology software from DNASTAR Inc., Madison, Wis.) was used to design three hybrid antimicrobial peptides (Vida1 to Vida3) with the aim of targeting pathogenic protozoa. The computer package allowed the study of the theoretical structure of each peptide, as well as polar and apolar orientations, space fill models, and charge distribution (R. B. G. Arrighi and J. G. Burgess, unpublished data).
Vida1 was the first hybrid designed and was used as a template for Vida2 and Vida3. Modifications made to Vida2 and Vida3 involved amino acid substitutions that would maintain the amphipathic nature and flexible region of the peptides while altering the predicted secondary structure (mainly helices in Vida1 to only sheets and coils in Vida3). The activities of these three novel peptides against Plasmodium spp. were compared with those of two template peptides, chosen on the basis of their predicted opposing secondary structures: P2WN, a peptide with a completely helical secondary structure (42), and ILF, a peptide with a completely random secondary structure (35).
Peptide synthesis and purification.
All designed and template amphiphilic peptides were synthesized by 9-fluorenylmethoxy carbonyl (Fmoc) chemistry with a solid-phase system with a P9050 Plus PepSynthesizer (Millipore, Bedford, Mass.). The synthesis resins Fmoc-l-Val-polyethylene glycol (PEG)-polystyrine (PS), Fmoc-l-Lys-PEG-PS, and Fmoc-l-Arg-PEG-PS were purchased from Applied Biosystems Japan Ltd. (Tokyo, Japan). The condensation reaction of Fmoc-protected amino acids was done by using O-(7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (Applied Biosystems Japan Ltd.) as an activator. After each synthesis reaction, the resins were washed, and then the synthesized peptides were released from the resins and were deblocked by trifluoroacetic acid treatment. The harvested crude peptides were purified by high-pressure liquid chromatography (Shimadzu, Kyoto, Japan) with a reverse-phase column (C18; 19 by 150 mm; Shimadzu).
Antiprotozoal peptide assays. (i) In vitro assay.
Parasitized blood containing gametocytes was obtained from infected mice (total parasitemia, ∼10%), passed through a CF11 column (Whatman) to remove white blood cells, and diluted 10 times in supplemented RPMI 1640 medium (32). The parasite culture was incubated at 19°C to initiate the sporogonic cycle. It was dispensed in 100-μl aliquots into the wells of microtiter plates at several time points after the initiation of culture (0, 2, 6, 10, 14, 18, and 24 h), and then 10 μl of antimicrobial peptide (final concentration, 50 μM in phosphate-buffered saline [PBS]) was immediately added to each well. The plates were incubated at 19°C until sampling, which was 6 h after the addition of each antimicrobial peptide. Parasites were observed and counted in a hemocytometer by phase-contrast light microscopy at ×400 magnification.
All five peptides (Vida1 to Vida3, P2WN, and ILF) and a control (PBS) were used individually to treat sets of wells containing P. berghei at each time point. Six wells were tested per peptide and per time point, and the experiment was repeated three times. Following this initial study, the two main candidates (in terms of the peptides with the highest levels of inhibitory activity), Vida3 and ILF, were tested against a different species of rodent malaria parasite, P. yoelii nigeriensis. Peptide activity was carried out and measured by the same protocol used for the tests with P. berghei.
(ii) In vivo assay.
Each antimicrobial peptide (final concentration, 50 μM) was mixed with freshly collected P. berghei-infected mouse blood containing exflagellating gametocytes, and the mixture was immediately fed to 3-day-old female A. gambiae mosquitoes through an artificial membrane feeder (Hemotek Membrane Feeding Systems, Accrington, United Kingdom). Fully engorged mosquitoes were transferred to an incubator and were maintained at ∼20°C for 10 days and provided with sugar solution ad libitum. The midguts of the mosquitoes were then dissected to check the parasite prevalence. A subsample of the mosquitoes in each treatment group was examined to determine the intensity of infection. The inhibitory activities of all the peptides were tested in this manner and compared with that of the PBS control. Each experiment was repeated three times.
Peptide injections.
Antimicrobial peptides (1 μl of a 50 μM stock) were injected into groups of naturally infected mosquitoes 4 days after a blood meal, when oocysts were present in the midgut. The same volume of PBS was injected into a control group. Solutions were injected into the thorax by using a finely drawn out calibrated glass microcapillary tube. The mosquitoes were maintained as described above and dissected after 10 days to check for oocysts. The experiment was repeated several times.
Statistical analysis.
All statistical analyses were carried out with the MINITAB computer program. None of the data were normally distributed (Anderson-Darling test); therefore, the Kruskal-Wallis test for nonparametric data was used to analyze overall differences. The Mann-Whitney U test was then applied to pairwise comparisons between different treatment groups. All P values between comparisons were adjusted by use of Holm's test, which adjusts the calculation of probability in line with the number of comparisons made. This helps to avoid type I errors that may occur as a result of making multiple pairwise comparisons (15).
Different levels of parasite prevalence between treatments were compared by chi-square tests.
RESULTS
Design of peptide with activities against parasitic protozoa.
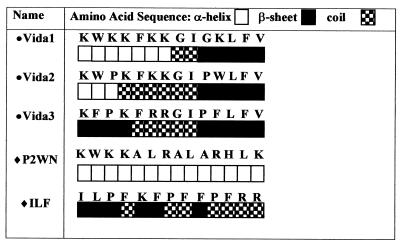
The predicted secondary structures of the novel peptides (Fig. 1) consisted mainly of α helices (Vida1), β sheets (Vida2), or a combination of coils and sheets (Vida3). The predicted secondary structures of the P2WN and ILF templates (completely helical and random, respectively; Fig. 1) were confirmed by circular dichroism analysis (35, 42).
FIG. 1.
Amino acid sequences and predicted secondary structure of novel and template hybrid antimicrobial peptides. The predicted secondary structures for novel hybrids range from mainly helical (Vida1), through a mixture of sheets and helices (Vida2), to mainly sheets and coils (Vida3). The template hybrids are predicted to have either a completely helical structure (P2WN) or a random structure (ILF). •, newly designed peptides; ⧫, template hybrid peptides (35, 42).
Antimicrobial peptides vary in their levels of activity.
A pilot study was carried out by testing the activities of all the short hybrids against Escherichia coli and Leishmania hertigi. Peptide activities were measured at 30 min and 24 h of growth of the two microbes, respectively. The differences in the numbers of E. coli CFU and in the numbers of viable L. hertigi promastigotes detected before and after treatment were measured. The results suggested that P2WN was most effective against bacteria but had little effect against the protozoan L. hertigi. In complete contrast, both Vida2 and Vida3 had little effect against E. coli but were far more active against L. hertigi (R. B. G. Arrighi, C. Nakamura, J. Miyake, and J. G. Burgess, unpublished data). This work supported our initial hypothesis that short peptides with activities against protozoa could be designed on the basis of their predicted secondary structures. Following this initial work we studied the effects of the novel peptides against sporogonic stages of the malaria parasite.
Hybrid peptides are active against the sporogonic forms of P. berghei (in vitro).
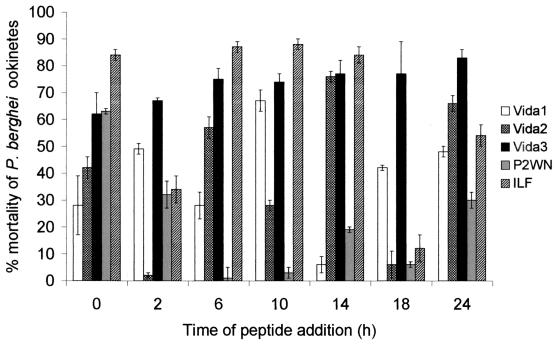
Each of the hybrid peptides tested had some effect on parasite mortality during the course of the experiment (Fig. 2). Since parasite development is asynchronous, the counts were based on those for the predominant parasite stage 6 h after addition of the peptide. While peptide activity is believed to be rapid, the counts were delayed for several hours to facilitate the observations. In our culture system, the timing of development is as follows: gamete fertilization and zygote formation, 0 to 3 h; retort-form ookinete formation, 5 to 9 h; young ookinete formation, 10 to 14 h; and mature ookinete formation, ≥15 h. Since it was not possible to distinguish between young and mature ookinetes, the forms observed were classified as predominantly retort-form ookinetes (between 0 and 2 h after peptide addition) and maturing ookinetes (≥6 h after peptide addition).
FIG. 2.
Mean percent reduction in P. berghei ookinetes following in vitro incubation with the novel and template peptides Vida1, Vida2, Vida3, P2WN, or ILF. The numbers of parasites were counted 6 h after peptide addition, and activity was expressed by comparison with the counts of control ookinetes (i.e., those in PBS with no peptide). Error bars represent the standard error of the mean (n = 18).
Some of the peptides were not active against all the different stages of P. berghei targeted. Vida1 produced rates of mortality of about 65% at 10 h (young ookinete) but had less of an effect at all other time points sampled. Vida2 was very effective at 14 and 24 h (maturing ookinetes), killing between 60 and 70% of the parasites present. P2WN was effective only against the first stages (0 h) of parasite development. In contrast, Vida3 caused a consistently high level of mortality (>60%) throughout the development period, and ILF was very effective at four of the time points studied (0, 6, 10, and 14 h).
Peptides are effective at targeting a different species of rodent malaria.
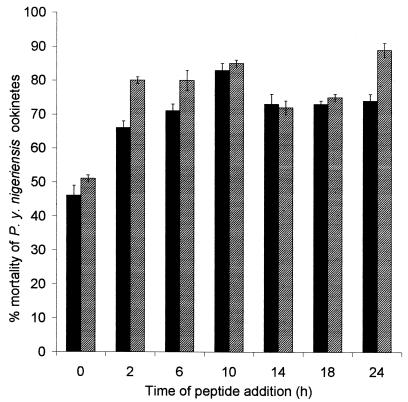
Since Vida3 and ILF showed the most consistently high levels of activity against the different stages of P. berghei, we tested their activities against a different species of rodent malaria, P. yoelii nigeriensis (Fig. 3). Both peptides again showed consistently high levels of activity, although the overall profiles were different from those of their activities against P. berghei. Vida3 and ILF were very active (causing 80% mortality) at every time point tested except 0 h.
FIG. 3.
Mean percent reduction in P. yoelii nigeriensis ookinetes following in vitro incubation with the novel peptides Vida3 (black bars) and ILF (striped bars). The parasites were counted 6 h after peptide addition, and activity was expressed by comparison with the counts of control ookinetes (i.e., those with no peptide). Error bars represent the standard error of the mean (n = 18).
Peptides exert a toxic effect against early-stage parasites in vivo.
All five hybrid peptides were tested against the early in vivo stages of P. berghei developing within A. gambiae (Table 1). Data from repeat experiments were pooled, as no significant difference was found between blocks for each treatment (P ≫ 0.05 by the Kruskal-Wallis test).
TABLE 1.
Antimicrobial activities of peptides against P. berghei in the mosquito vector A. gambiae following feeding through an artificial membranea
| Peptide treatment | Intensity of infection
|
Prevalence of infection
|
||
|---|---|---|---|---|
| No. of mosquitoes sampled | Median no. of oocysts/midgut (lower/upper quartile range) | No. of mosquitoes sampled | % infected mosquitoes | |
| Vida1 | 23 | 34 (28/37.5)b | 43 | 84 |
| Vida2 | 27 | 27 (8/34)b | 63 | 79 |
| Vida3 | 33 | 2 (0/4)c | 53 | 62d |
| P2WN | 24 | 33 (29.75/40.5)b | 57 | 84 |
| ILF | 25 | 7 (5/9)e | 48 | 62d |
| None (PBS) | 23 | 61 (53.5/65) | 39 | 87 |
The blood was mixed with peptide or PBS prior to feeding. The midguts were dissected 10 days after the blood meal and checked for oocysts in order to measure prevalence and parasite intensity. All P values were calculated by the Mann-Whitney U test with Holm's adjustment.
Significantly different (P < 0.0001) from value for oocysts found in mosquitoes fed a PBS-treated blood meal.
Significantly different (P < 0.001) from the values for all other treatments.
Significant decrease (P < 0.05) in the prevalence of infection compared to that in mosquitoes fed a PBS-treated blood meal.
Significantly different (P < 0.0001) from the values for all other treatments except Vida3.
Almost all mosquitoes were still infected with at least one oocyst after ingestion of the Vida1, Vida2, and P2WN peptides; and the overall prevalence of mosquitoes that were infected (Table 1) did not differ significantly between the treatment groups (P = 0.405 by the chi-square test). However, there were significant decreases in the prevalence of infection in the Vida3- and ILF-treated groups compared with that in the control group of mosquitoes (P = 0.04 and P = 0.044, respectively, by the chi-square test). Approximately 38% of mosquitoes that were fed an infected blood meal to which either Vida3 or ILF was added did not develop any oocysts.
The addition of the antimicrobial peptides caused an overall significant reduction in the number of oocysts formed within the mosquitoes (P < 0.0001 by the Kruskal-Wallis test) (Table 1). Each of the peptides caused a significant reduction in the number of oocysts formed compared to the number of oocysts formed in mosquitoes fed a PBS-treated blood meal (P < 0.0001 by the Mann-Whitney U test). Vida3 and ILF each reduced the number of oocysts by approximately 90% (P < 0.0001 by the Mann-Whitney U test), and Vida3 caused significantly more parasite mortality than ILF (P = 0.001 by the Mann-Whitney U test).
Specific hybrid peptides are very active against oocysts.
The second in vivo experiment tested the activities of the peptides against oocysts rather than the early stages of infection (zygotes, ookinetes, etc.), as the peptides were injected after oocyst formation. As there was no significant difference between any of the blocks of data for each treatment, the data were again pooled (P ≫ 0.05 by the Kruskal-Wallis test). Injection of the antimicrobial peptides resulted in an overall significant drop in the oocyst burden (P < 0.0001 by the Kruskal-Wallis test). Unlike the experiment in which the mosquitoes were fed through an artificial membrane, the prevalence of infection of infected mosquitoes did not differ significantly between any of the treatment groups and the group of mosquitoes injected with PBS (P = 0.619 by the chi-square test) (Table 2). The Vida3 and ILF treatments significantly reduced the oocyst burdens compared with those after PBS treatment (P = 0.0018 and P = 0.0045, respectively, by the Mann-Whitney test). In contrast to its activity against early stages, Vida3 did not cause more parasite mortality than ILF (P = 0.0848 by the Mann-Whitney U test).
TABLE 2.
Antimicrobial activities of peptides against P. berghei in the mosquito vector A. gambiaea
| Peptide treatment | No. of mosquitoes sampled | Median no. of oocysts/ midgut (lower/upper quartile range) | Prevalence of infected mosquitoes (%) |
|---|---|---|---|
| Vida1 | 24 | 27.5 (12/37.25) | 75 |
| Vida2 | 28 | 27 (23.5/34) | 86 |
| Vida3 | 40 | 4 (0/8.25)b | 65 |
| P2WN | 26 | 34.5 (30.25/38) | 77 |
| ILF | 24 | 1.5 (0/3)b | 58 |
| None (PBS) | 25 | 32 (0/38) | 72 |
Mosquitoes were allowed to feed on an infected mouse. Four days after the blood meal, each mosquito was treated via injection of either a peptide or PBS into the hemolymph. The midguts were dissected 10 days after the blood meal and checked for oocysts in order to measure parasite prevalence and intensity.
Significantly different (P = 0.0018 and P = 0.0045 for Vida3 and ILF, respectively) from the value for PBS-treated mosquitoes, as calculated by the Mann-Whitney U test with Holm's adjustment.
DISCUSSION
The objective of this work was to produce novel peptides with high levels of activity against parasitic protozoa. This has been achieved through the design and application of highly effective hybrid peptides against malaria parasites that are based on naturally occurring and synthetic peptides. Although our novel hybrids, Vida1 to Vida3, were identical in length, they varied in their amino acid sequences and were predicted to have a range of secondary structures. Vida3 and ILF, the novel and template peptides, respectively, that proved to be the most active against the sporogonic forms of two species of rodent malaria parasites had predicted secondary structures that were very different from those of the other hybrids. They contained the highest contents of random turns and coils and low contents of β sheets and no α helices (Fig. 1). This study thus supports our initial hypothesis that differences in secondary structure may contribute to variations in activity against different types of microorganisms.
Antimicrobial peptides are thought to form pores in their target lipid bilayer, resulting in depolarization, which leads to a rapid influx of solutes. Although it is thought that antimicrobial peptides need to span membranes in order to form pores, studies have shown that other mechanisms exist by which antimicrobial peptides may exert their effects on a target membrane (6). It is possible that our short novel peptides use one of these alternative modes of action.
The in vitro studies with P. berghei showed that each of the peptides tested had some killing effect on the parasites. Moreover, different peptides had different profiles of activity over the 24-h period of gamete to ookinete development. This is likely the result of the fact that each peptide interacts with different lipid composites on the parasite surface and the profiles of these composites may change as the parasite develops (9, 18). Expression of different lipids or proteins can alter the surface charges of cells. It has been shown that factors such as hydrophobicity and the distribution of the charge contribute enormously to the activities of antimicrobial peptides (6, 16). This may be the case for the novel peptides designed in this study. By using artificially constructed liposomes filled with a fluorescent dye, the rate of dye release following incubation with our novel and template hybrids was shown to change dramatically depending on the surface charge with which they are interacting (Arrighi et al., unpublished data).
Surprisingly, the time profiles of the activities of Vida3 and ILF against P. yoelii nigeriensis differed from those observed against P. berghei, suggesting that the surface membrane ontogenies of these closely related species may differ.
The peptides used in this study may cause cell lysis, as the number of visible parasites in each of the peptide treatment groups was lower than that in the control (PBS) treatment group. In addition, several remaining ookinetes were swollen, a response consistent with the process of antimicrobial activity.
When assessing the potential of Vida3 for use as an agent against malaria parasite sporogony, several factors should be considered. First, conditions in the mosquito midgut lumen were not detrimental toward peptide activity, possibly because midgut protease activity does not peak until 24 h after intake of a blood meal (17). Second, the activity of the peptide against oocysts was an unexpected bonus. Oocysts developing beneath the midgut basal lamina are thought to be in a privileged site and protected from the hemolymph antimicrobial peptides by a capsule composed, in part, of components of the basal lamina (1). Unlike the midgut and sporozoite stages, little parasite death occurs naturally at this stage (37, 38). Our findings suggest that the small peptide Vida3 can gain access to the developing oocyst via the hemocoel.
Malaria sporozoites are known to be vulnerable to some naturally occurring antimicrobial peptides (31). If this proves to be the case for the novel peptide Vida3, engineering of its continued expression and secretion into the mosquito gut lumen and hemolymph would provide an opportunity to express its activity against all sporogonic stages. Such long-term exposure is likely to result in a cumulative effect and thus to improve upon the 80% reduction in intensity of infection and the 25% reduction in prevalence already observed in P. berghei-infected A. gambiae mosquitoes in these experiments. If this peptide is effective against a human malaria-mosquito association such as P. falciparum-A. gambiae, it could considerably reduce the rates of parasite transmission.
However, we must consider the possible toxic effect that the cumulative effect of peptide production may have on the mosquito. Studies that administered magainins and cecropins to infected A. gambiae mosquitoes found that the peptides were toxic to the mosquitoes when the peptides were used at levels between 0.05 and 0.5 μg/μl (14).
The peptide concentration used throughout our experiments is comparable to the antimicrobial peptide levels reported in some insects following injury or infection (20), and the concentrations that we injected would be considerably diluted by the hemolymph. A lack of material precluded a dose-response experiment, so we were unable to determine whether higher concentrations would destroy all parasites. Long-term strategies for disease control in vectors are concentrating on genetic modification of the symbiotic flora of disease vectors to express these peptides (5) or the production of transgenic mosquitoes which express antimicrobial peptides (11, 27). The peptides could be expressed at specific times (i.e., during blood meal digestion or invasion) or continuously. Candidates for expression include defensin (19, 31) and Shiva-3, a synthetic cecropin-like peptide (27). The upregulation of naturally occurring antimicrobial peptides must, however, be viewed with some caution until the fitness costs of this strategy involving mosquitoes are fully understood. It has been demonstrated that the reproductive fitness of A. gambiae is significantly impaired when the humoral immune system is stimulated (2). We would need to test the effects of novel, synthetic antimicrobial peptides on reproductive fitness before their introduction into the mosquito genome is considered as a control strategy.
Antimicrobial peptides may also have an indirect effect on malaria parasite survival. For example, some synthetic peptides have been shown to kill intracellular blood-stage forms of the malaria parasite (13). In addition, some studies have shown that antimicrobial peptides can induce cells to undergo apoptosis (30). It has been shown that approximately 50% of P. berghei ookinetes undergo apoptosis in the mosquito midgut (E. Al-Olayan, G. Williams, and H. Hurd, submitted for publication). Although the apoptosis trigger has not been identified, defense peptides are contenders. Other work suggests that antimicrobial peptides can trigger nitric oxide synthase expression (39). Nitric oxide synthase is upregulated during malaria parasite infection, and NO has been shown to limit Plasmodium development in vivo (24).
The application of short novel antimicrobial peptides such as Vida3 against vector-transmitted protozoan parasites has a variety of advantages over the application of natural and longer synthetic hybrid peptides. The specificity of the design appears to increase activity. Small size facilitates prospects for both large-scale synthetic production and natural expression in a genetically modified mosquito. A precedence for the latter has already been demonstrated with a genetically modified tobacco plant, which expressed a synthetic antimicrobial peptide active against pathogenic fungi (10).
More work is required to design and test short novel antibacterial peptides that completely inhibit malaria parasite transmission, but our study suggests that this approach has potential.
Acknowledgments
We thank Ann Underhill for insectary assistance. In addition, we thank members of the Tissue Engineering Research Center for assistance and comments and Ashley Scott (Tissue Engineering Research Center) for pilot project work.
R. Arrighi was funded by a Departmental Research Studentship from the School of Life Sciences, Keele University.
REFERENCES
- 1.Adini, A., and A. Warburg. 1999. Interaction of Plasmodium gallinaceum and oocysts with extracellular matrix proteins. Parasitology 119:331-336. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, A. M., S. L. Baggott, R. Maingon, and H. Hurd. The costs of mounting an immune response are reflected in the reproductive fitness of the mosquito Anopheles gambiae. Oikos, in press.
- 3.Al-Olayan, E. M., A. L. Beetsma, G. A. Butcher, R. E. Sinden, and H. Hurd. 2002. Complete development of mosquito phases of the malaria parasite in vitro. Science 295:677-679. [DOI] [PubMed] [Google Scholar]
- 4.Andreu, D., J. Ubach, A. Boman, B. Wahlin, D. Wade, R. B. Merrifield, and H. G. Boman. 1992. Shortened cecropin A-melittin hybrids: significant size reduction retains potent antibiotic activity. FEBS Lett. 296:190-194. [DOI] [PubMed] [Google Scholar]
- 5.Beard, C. B., E. M. Dotson, P. M. Pennington, S. Eichler, C. Cordon-Rosales, and R. V. Durvasula. 2001. Bacterial symbiosis and paratransgenic control of vector-borne Chagas disease. Int. J. Parasitol. 31:621-627. [DOI] [PubMed] [Google Scholar]
- 6.Bechinger, B. 1999. The structure, dynamics and orientation of antimicrobial peptides in membranes by multidimensional solid-state NMR spectroscopy. Biochim. Biophys. Acta 1462:157-183. [DOI] [PubMed] [Google Scholar]
- 7.Boman, H. G., and D. Hultmark. 1987. Cell-free immunity in insects. Annu. Rev. Microbiol. 41:103-126. [DOI] [PubMed] [Google Scholar]
- 8.Bulet, P., C. Hetru, J. L. Dimarcq, and D. Hoffman. 1999. Antimicrobial peptides in insects: structure and function. Dev. Comp. Immunol. 23:329-344. [DOI] [PubMed] [Google Scholar]
- 9.Carter, R., and D. C. Kaushal. 1984. Characterization of antigens on mosquito midgut stages of Plasmodium gallinaceum. III. Changes in zygote surface proteins during transformation to mature ookinete. Mol. Biochem. Parasitol. 13:235-241. [DOI] [PubMed] [Google Scholar]
- 10.Cary, J. W., K. Rajasekaran, J. M. Jaynes, and T. E. Cleveland. 2000. Transgenic expression of a gene encoding a synthetic antimicrobial peptide results in inhibition of fungal growth in vitro and in planta. Plant Sci. 154:171-181. [DOI] [PubMed] [Google Scholar]
- 11.Conde, R., F. Z. Zamudio, M. H. Rodriguez, and L. D. Possani. 2000. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 471:165-168. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Lopez, S., H. S. Kim, E. C. Choi, M. Delgado, J. R. Granja, A. Khashanov, K. Kraehenbuehl, G. Long, D. A. Weinberger, K. M. Wilcoxen, and M. R. Ghadiri. 2001. Antibacterial agents based on the cyclic d,l-α-peptide architecture. Nature 412:452-455. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh, J. K., D. Shaool, P. Guillaud, L. Ciceron, D. Mazier, I. Kustanovich, Y. Shai, and A. Mor. 1997. Selective cytotoxicity of dermaseptin S3 toward intraerythrocytic Plasmodium falciparum and the underlying molecular basis. J. Biol. Chem. 272:31609-31616. [DOI] [PubMed] [Google Scholar]
- 14.Gwadz, R. W., D. Kaslow, J. Y. Lee, W. L. Maloy, M. Zasloff, and L. H. Miller. 1989. Effects of magainins and cecropins on the sporogonic development of malaria parasites in mosquitoes. Infect. Immun. 57:2628-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holm, S. 1979. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6:65-70. [Google Scholar]
- 16.Hwang, P. M., and H. J. Vogel. 1998. Structure-function relationships of antimicrobial peptides. Biochem. Cell Biol. 76:235-246. [DOI] [PubMed] [Google Scholar]
- 17.Jahan, N., and H. Hurd. 1997. The effects of infection with Plasmodium yoelii nigeriensis on the reproductive fitness of Anopheles stephensi. Ann. Trop. Med. Parasitol. 91:365-369. [DOI] [PubMed] [Google Scholar]
- 18.Kaushal, D. C., and R. Carter. 1984. Characterization of antigens on mosquito midgut stages of Plasmodium gallinaceum. II. Comparison of surface antigens of male and female gametes and zygotes. Mol. Biochem. Parasitol. 11:145-156. [DOI] [PubMed] [Google Scholar]
- 19.Kokoza, V., A. Ahmed, W. L. Cho, N. Jasinskiene, A. A. James, and A. Raikhel. 2000. Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti. Proc. Natl. Acad. Sci. USA 97:9144-9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowenberger, C. 2001. Innate immune response of Aedes aegypti. Insect Biochem. Mol. Biol. 31:219-229. [DOI] [PubMed] [Google Scholar]
- 21.Lowenberger, C., P. Bulet, M. Charlet, C. Hetru, B. Hodgeman, B. M. Christensen, and J. A. Hoffmann. 1995. Insect immunity: isolation of three novel inducible antibacterial defensins from the vector mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 25:867-873. [DOI] [PubMed] [Google Scholar]
- 22.Lowenberger, C. A., S. Kamal, J. Chiles, S. Paskewitz, P. Bulet, J. A. Hoffmann, and B. M. Christensen. 1999. Mosquito-Plasmodium interactions in response to immune activation of the vector. Exp. Parasitol. 91:59-69. [DOI] [PubMed] [Google Scholar]
- 23.Lowenberger, C. A., C. T. Smartt, P. Bulet, M. T. Ferdig, D. W. Severson, J. A. Hoffmann, and B. M. Christensen. 1999. Insect immunity: molecular cloning, expression, and characterization of cDNAs and genomic DNA encoding three isoforms of insect defensin in Aedes aegypti. Insect Mol. Biol. 8:107-118. [DOI] [PubMed] [Google Scholar]
- 24.Luckhart, S., Y. Vodovotz, L. Cui, and R. Rosenberg. 1998. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl. Acad. Sci. USA 95:5700-5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oduol, F., J. Xu, O. Niare, R. Natarajan, and K. D. Vernick. 2000. Genes identified by an expression screen of the vector mosquito Anopheles gambiae display differential molecular immune response to malaria parasites and bacteria. Proc. Natl. Acad. Sci. USA 97:11397-11402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters, W., and A. E. Ramkaran. 1980. The chemotherapy of rodent malaria. XXXII. The influence of p-aminobenzoic acid on the transmission of Plasmodium yoelii and P. berghei by Anopheles stephensi. Ann. Trop. Med. Parasitol. 74:275-282. [PubMed] [Google Scholar]
- 27.Possani, L. D., M. Zurita, M. Delepierre, F. H. Hernandez, and M. H. Rodriguez. 1998. From noxiustoxin to Shiva-3, a peptide toxic to the sporogonic development of Plasmodium berghei. Toxicon 36:1683-1692. [DOI] [PubMed] [Google Scholar]
- 28.Richman, A. M., P. Bulet, C. Hetru, C. Barillas-Mury, J. A. Hoffmann, and F. C. Kafatos. 1996. Inducible immune factors of the vector mosquito Anopheles gambiae: biochemical purification of a defensin antibacterial peptide and molecular cloning of preprodefensin cDNA. Insect Mol. Biol. 5:203-210. [DOI] [PubMed] [Google Scholar]
- 29.Richman, A. M., G. Dimopoulos, D. Seeley, and F. C. Kafatos. 1997. Plasmodium activates the innate immune response of Anopheles gambiae mosquitoes. EMBO J. 16:6114-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Risso, A., M. Zanetti, and R. Gennaro. 1998. Cytotoxicity and apoptosis mediated by two peptides of innate immunity. Cell. Immunol. 189:107-115. [DOI] [PubMed] [Google Scholar]
- 31.Shahabuddin, M., I. Fields, P. Bulet, J. A. Hoffmann, and L. H. Miller. 1998. Plasmodium gallinaceum: differential killing of some mosquito stages of the parasite by insect defensin. Exp. Parasitol. 89:103-112. [DOI] [PubMed] [Google Scholar]
- 32.Sinden, R. E., R. H. Hartley, and L. Winger. 1985. The development of Plasmodium ookinetes in vitro: an ultrastructural study including a description of meiotic division. Parasitology 91:227-244. [DOI] [PubMed] [Google Scholar]
- 33.Sinden, R. E. 1997. Infection of mosquitoes with rodent malaria, p. 67-91. In J. M. Crampton, C. B. Beard, and C. Louis (ed.), Molecular biology of insect disease vectors: a method manual. Chapman & Hall, London, United Kingdom.
- 34.Sinden, R. E. 1999. Plasmodium differentiation in the mosquito. Parassitologia 41:139-148. [PubMed] [Google Scholar]
- 35.Subbalakshmi, C., V. Krishnakumari, R. Nagaraj, and N. Sitaram. 1996. Requirements for antibacterial and hemolytic activities in the bovine neutrophil derived 13-residue peptide indolicidin. FEBS Lett. 395:48-52. [DOI] [PubMed] [Google Scholar]
- 36.Subbalakshmi, C., R. Nagaraj, and N. Sitaram. 1999. Biological activities of C-terminal 15-residue synthetic fragment of melittin: design of an analog with improved antibacterial activity. FEBS Lett. 448:62-66. [DOI] [PubMed] [Google Scholar]
- 37.Vaughan, J. A., B. H. Noden, and J. C. Beier. 1992. Population dynamics of Plasmodium falciparum sporogony in laboratory-infected Anopheles gambiae. J. Parasitol. 78:716-724. [PubMed] [Google Scholar]
- 38.Vaughan, J. A., L. Hensley, and J. C. Beier. 1994. Sporogonic development of Plasmodium yoelii in five anopheline species. J. Parasitol. 80:674-681. [PubMed] [Google Scholar]
- 39.Velasco, M., M. J. M. Diaz-Guerra, P. Diaz-Achirica, D. Andreu, L. Rivas, and L. Bosca. 1997. Macrophage triggering with cecropin A and melittin-derived peptides induces type II nitric oxide synthase expression. J. Immunol. 158:4437-4443. [PubMed] [Google Scholar]
- 40.Vizioli, J., P. Bulet, M. Charlet, C. Lowenberger, C. Blass, H.-M. Muller, G. Dimopoulos, J. Hoffmann, F. C. Kafatos, and A. Richman. 2000. Cloning and analysis of a cecropin gene from the malaria vector mosquito. Anopheles gambiae. Insect Mol. Biol. 9:75-84. [DOI] [PubMed] [Google Scholar]
- 41.Vizioli, J., P. Bulet, J. A. Hoffmann, F. C. Kafatos, H. M. Muller, and G. Dimopoulos. 2001. Gambicin: a novel immune responsive antimicrobial peptide from the malaria vector Anopheles gambiae. Proc. Natl. Acad. Sci. USA 98:12630-12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong, L., R. J. Putnam, W. C. Johnson, Jr., and A. G. Rao. 1995. Design and synthesis of amphipathic antimicrobial peptides. Int. J. Protein Res. 45:337-347. [DOI] [PubMed] [Google Scholar]