Abstract
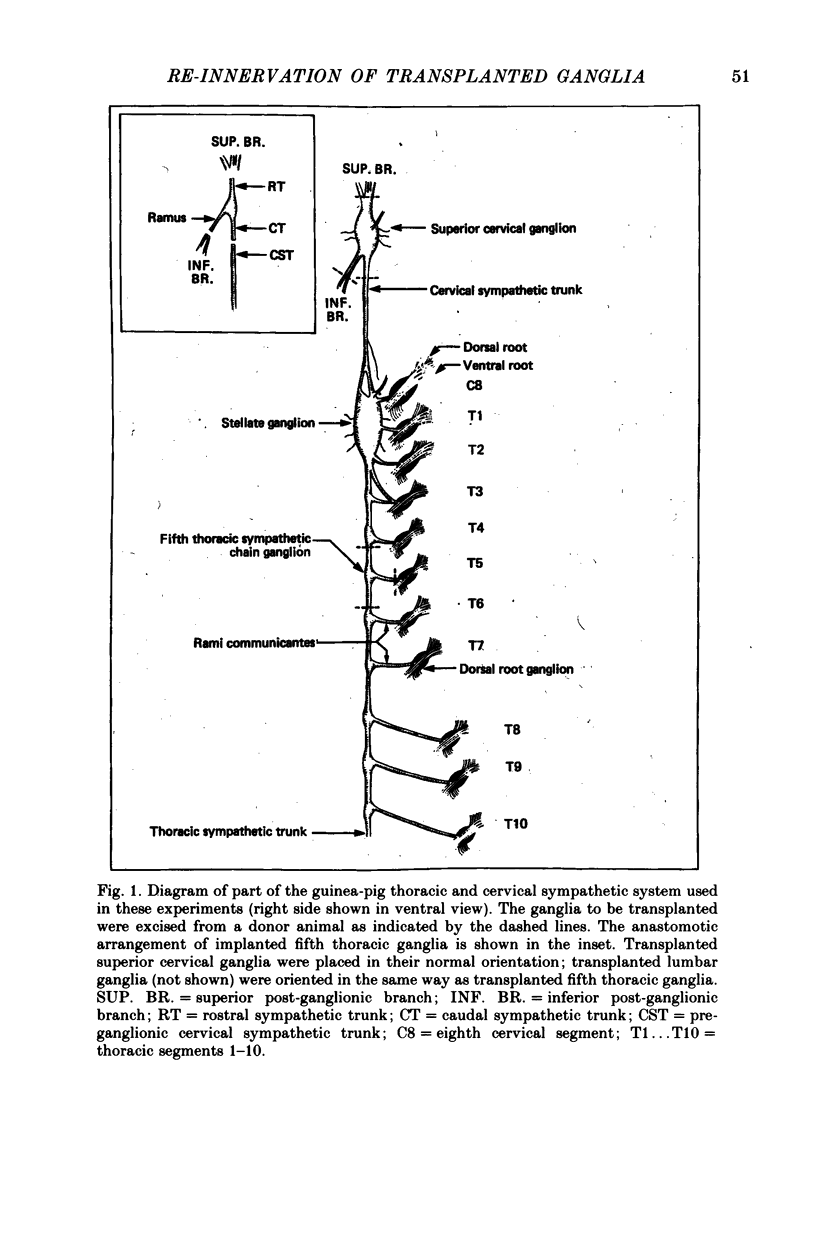
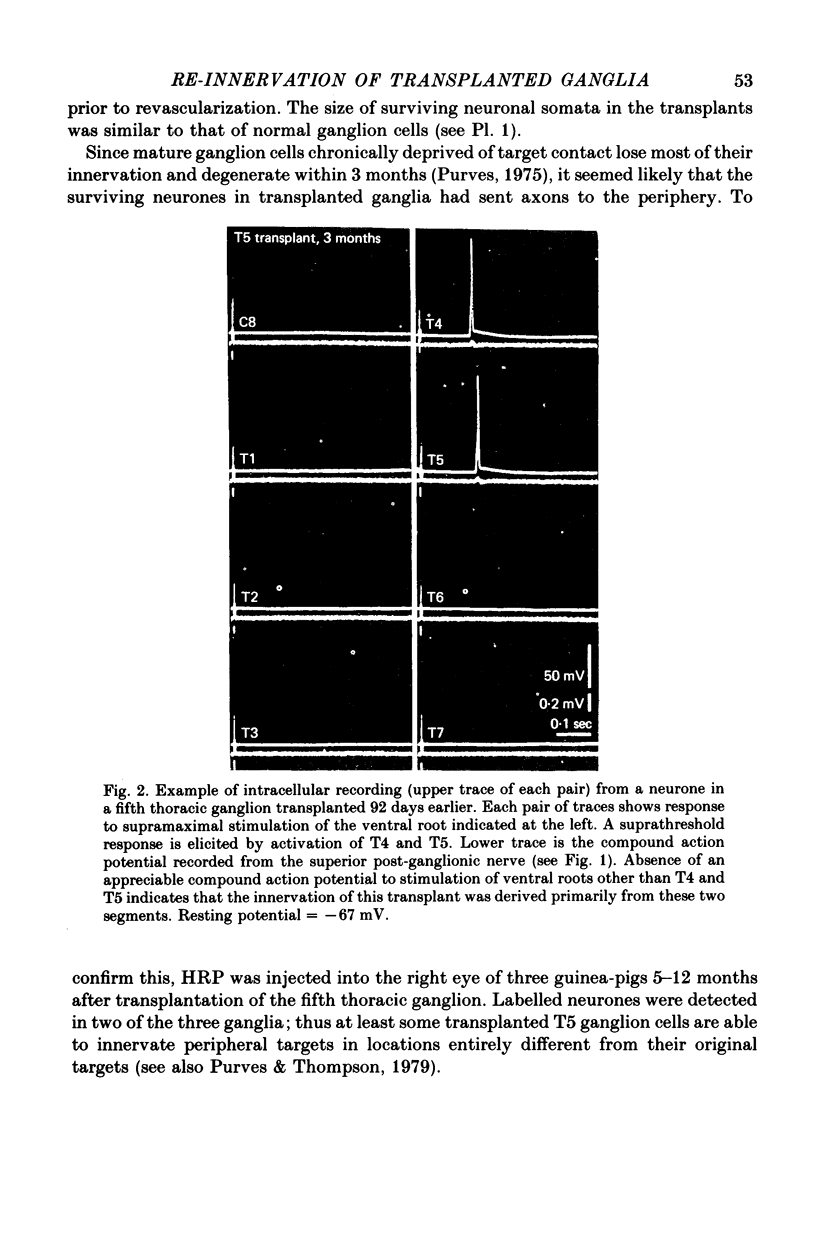
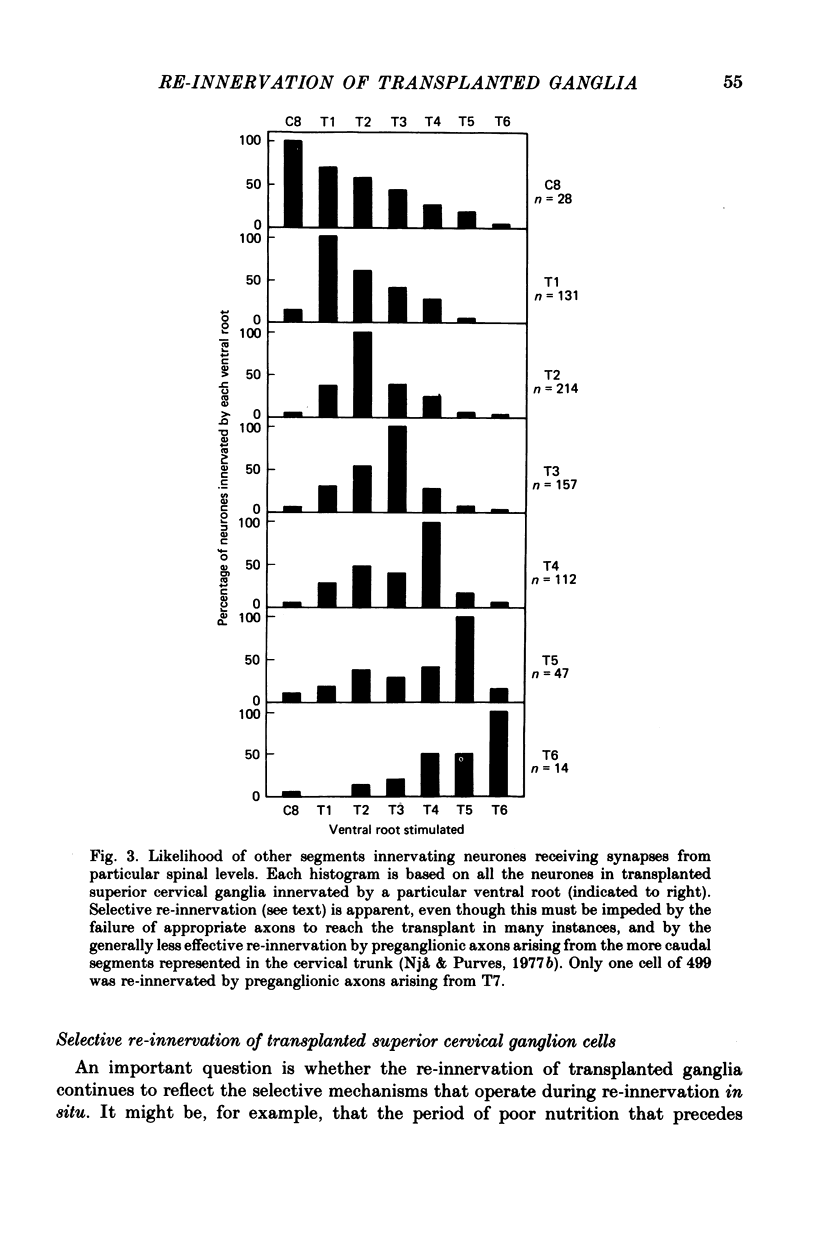
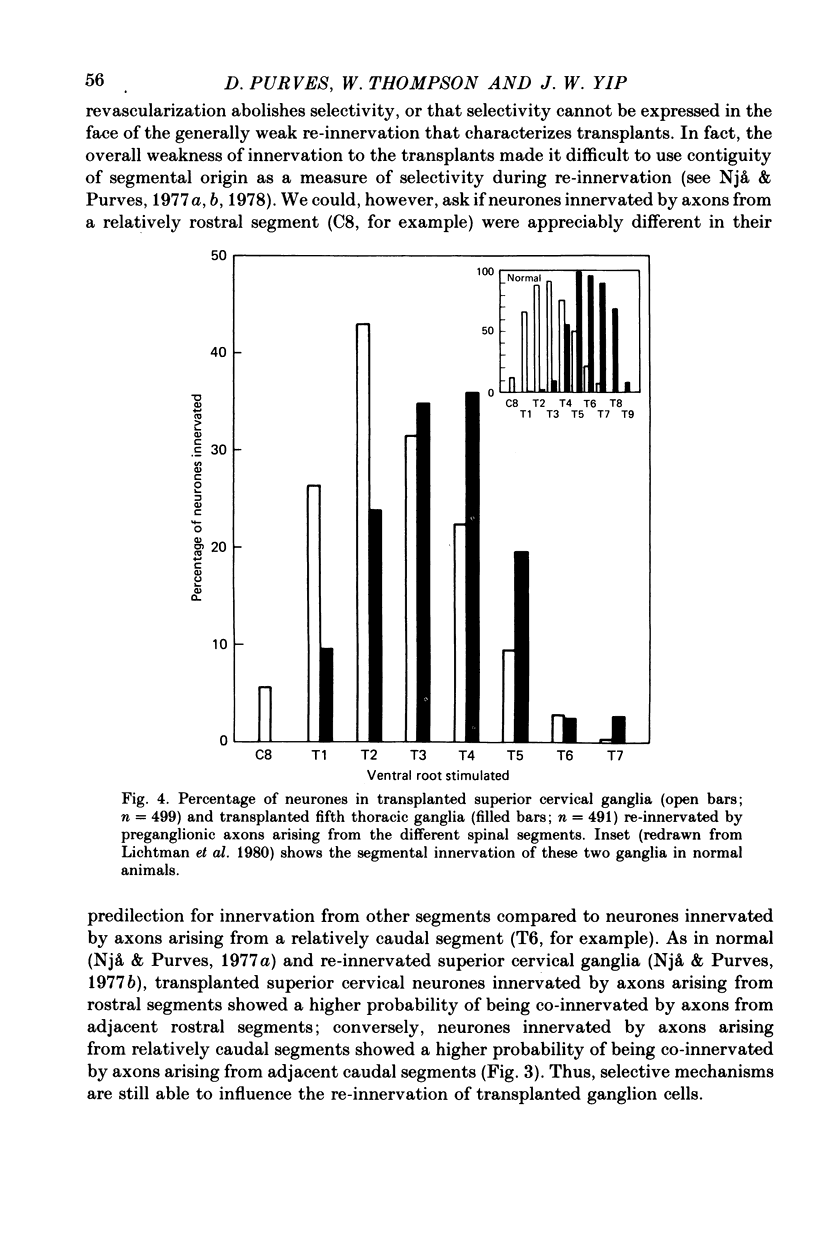
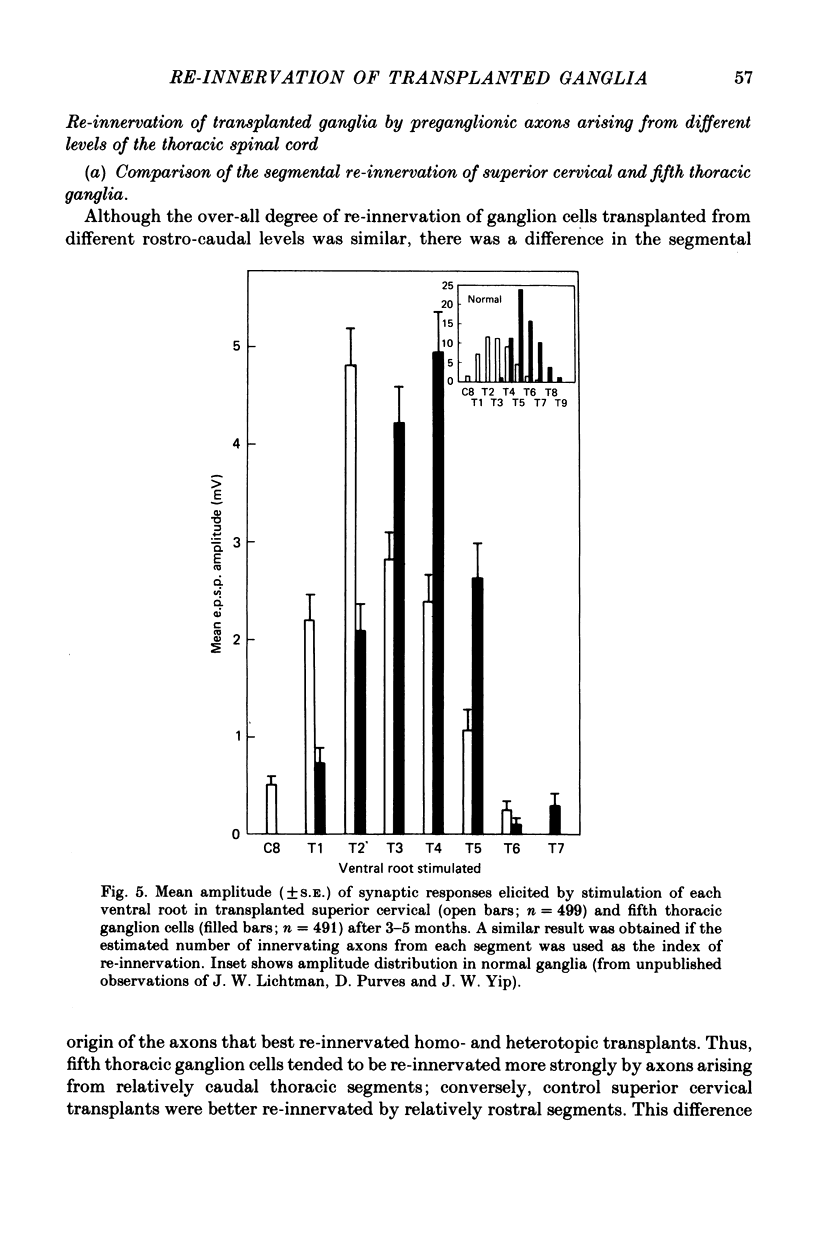
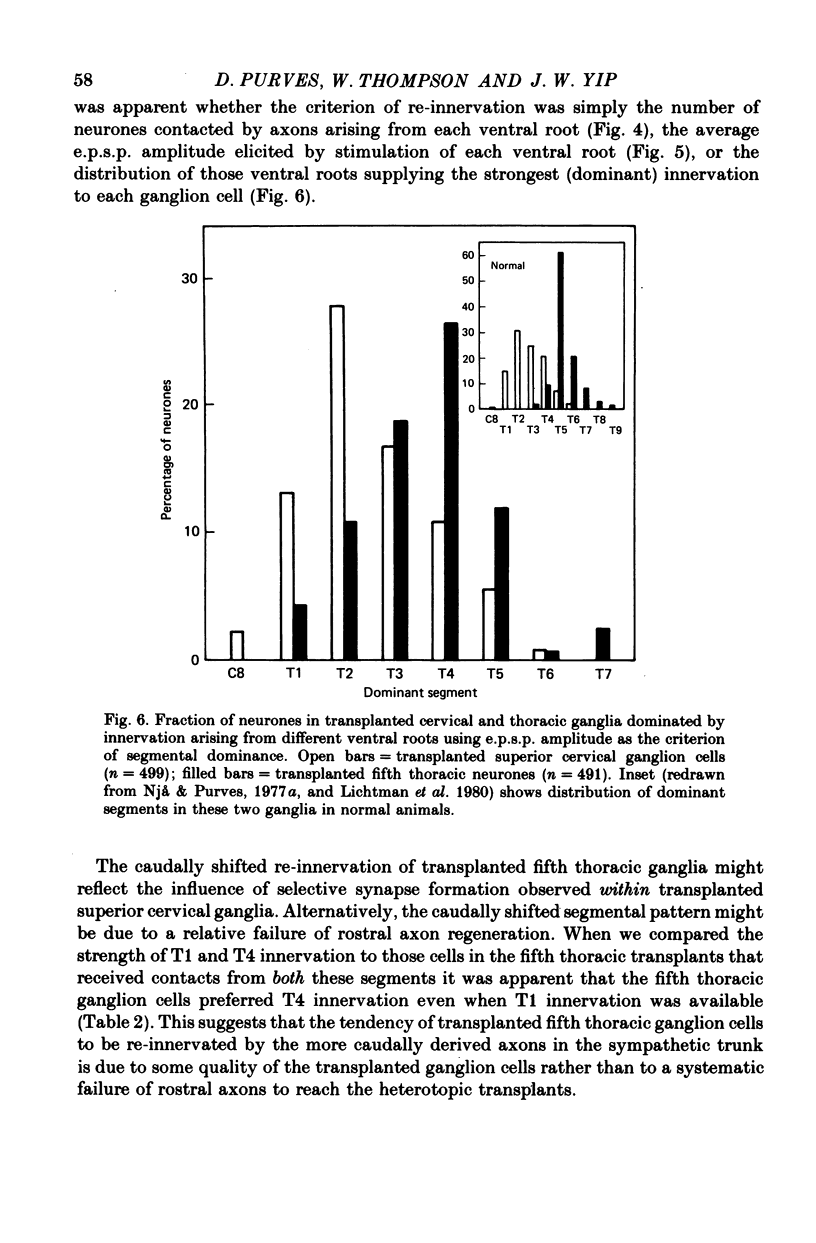
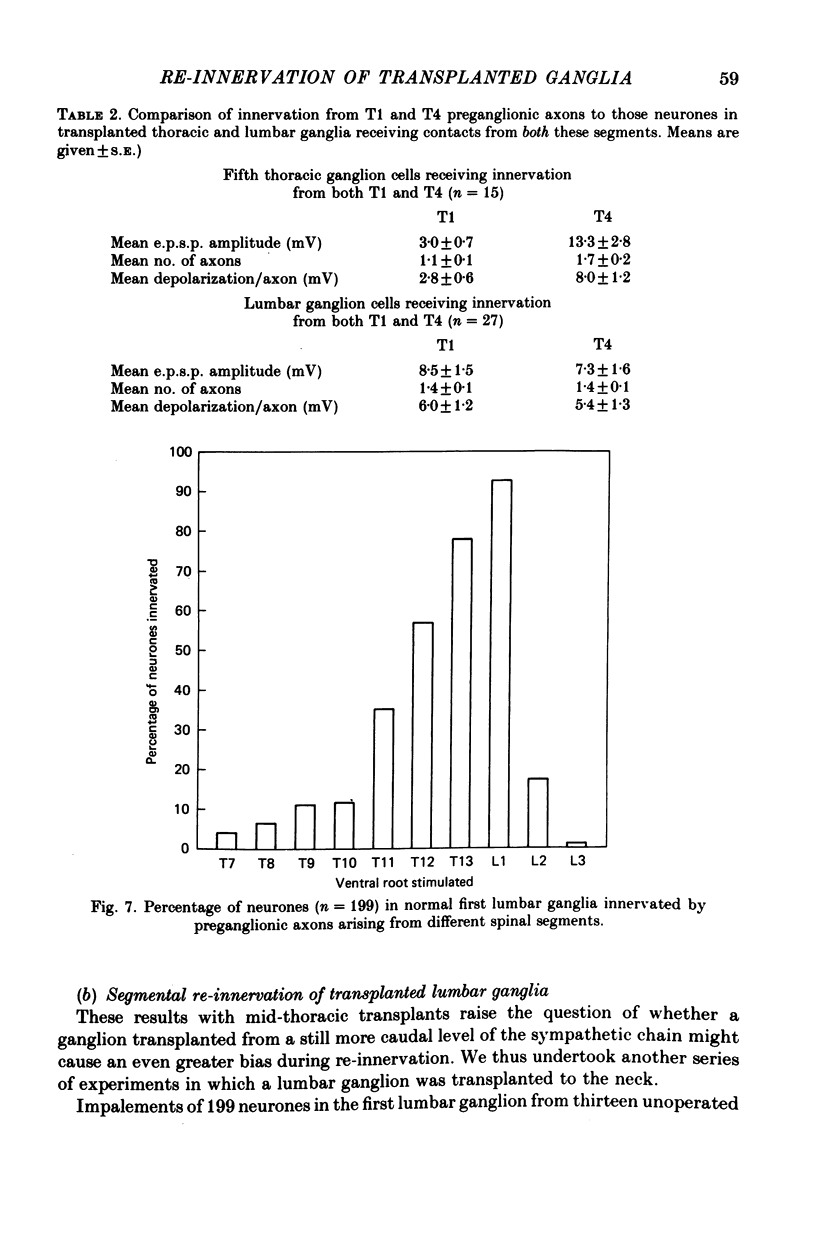
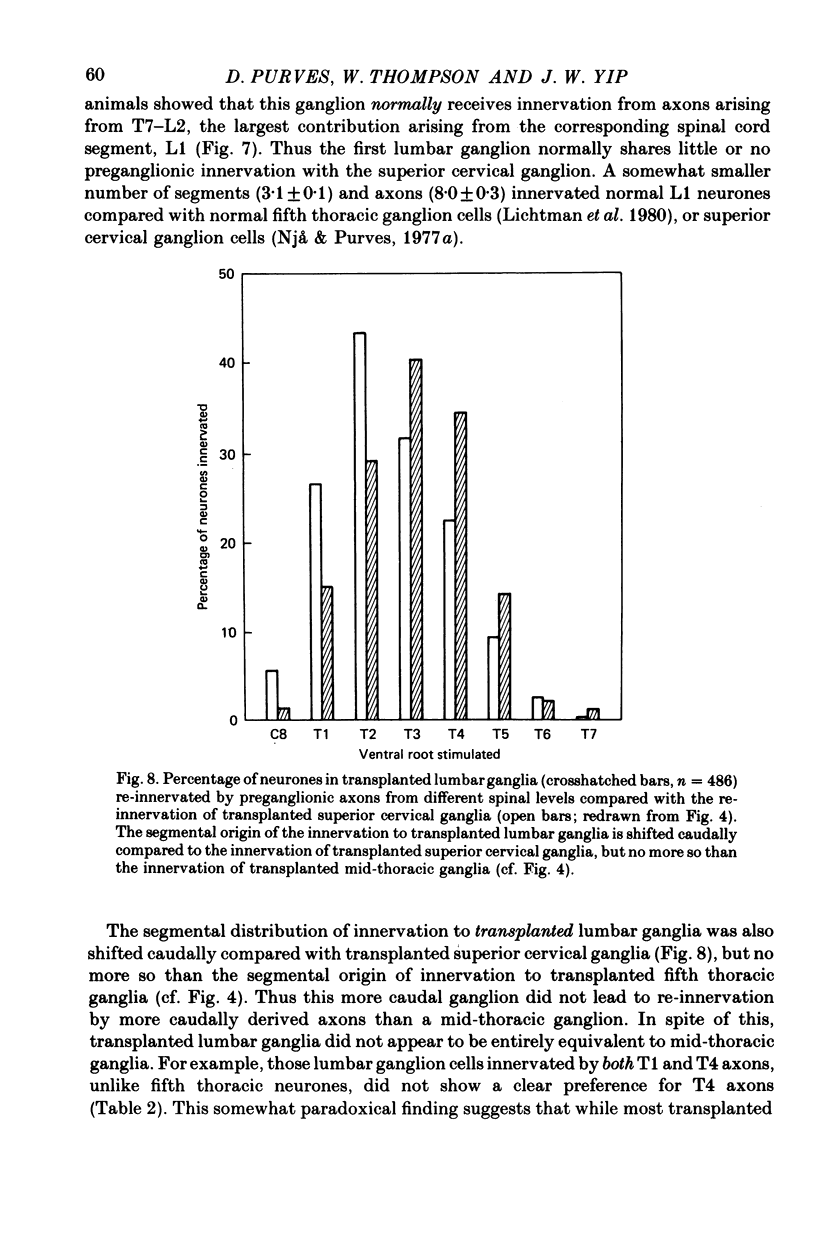
Thoracic and lumbar sympathetic ganglia from donor guinea-pigs were transplanted to the bed of an excised superior cervical ganglion in host animals. Homotopic transplants of superior cervical ganglia served as controls. In this way the same set of preganglionic axons (the cervical sympathetic trunk) was confronted with ganglia from different levels of the sympathetic chain. Re-innervation of the transplants was studied after 3-5 months. 1. Neurones in ganglia transplanted from different levels of the sympathetic chain were re-innervated to about the same over-all degree by the preganglionic axons of the host's cervical sympathetic trunk. Thus, the mean amplitude of post-synaptic potentials, the estimated number of innervating axons, and the number of spinal segments providing innervation to each neurone were similar in transplanted thoracic, lumbar and superior cervical ganglion cells. 2. Neurones in transplanted mid-thoracic ganglia, however, were re-innervated more frequently, and more strongly, by axons arising from more caudal thoracic segments than neurones in transplanted superior cervical ganglia. Stimulation of axons arising from the fourth thoracic spinal segment (T4), for example, elicited post-synaptic potentials that on average were twice as large in transplanted fifth thoracic ganglion cells as in transplanted superior cervical ganglion cells; conversely, axons arising from T1 re-innervated neurones in the superior cervical ganglion about 2-3 times more effectively than fifth thoracic ganglion cells. This difference in the re-innervation of the fifth thoracic and the superior cervical ganglion is in the same direction as (although less pronounced than) the normal difference in the segmental innervation of these ganglia. 3. Transplanted lumbar ganglia were also re-innervated more effectively by relatively caudal segments compared to re-innervated cervical ganglia, but this difference was no greater than that observed for transplanted thoracic ganglia. 4. We conclude that preganglionic axons can distinguish (or be distinguished by) ganglia derived from different levels of the sympathetic chain. Our findings are consistent with the view that ganglion cells have some permanent property that biases the innervation they receive.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Langley J. N. Note on Regeneration of Prae-Ganglionic Fibres of the Sympathetic. J Physiol. 1895 Jul 18;18(3):280–284. doi: 10.1113/jphysiol.1895.sp000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D., Yip J. W. Innervation of sympathetic neurones in the guinea-pig thoracic chain. J Physiol. 1980 Jan;298:285–299. doi: 10.1113/jphysiol.1980.sp013081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D., Yip J. W. On the purpose of selective innervation of guinea-pig superior cervical ganglion cells. J Physiol. 1979 Jul;292:69–84. doi: 10.1113/jphysiol.1979.sp012839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nja A., Purves D. Re-innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Nov;272(3):633–651. doi: 10.1113/jphysiol.1977.sp012064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. Specific innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Jan;264(2):565–583. doi: 10.1113/jphysiol.1977.sp011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. Specificity of initial synaptic contacts made on guinea-pig superior cervical ganglion cells during regeneration of the cervical sympathetic trunk. J Physiol. 1978 Aug;281:45–62. doi: 10.1113/jphysiol.1978.sp012408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Formation and maintenance of synaptic connections in autonomic ganglia. Physiol Rev. 1978 Oct;58(4):821–862. doi: 10.1152/physrev.1978.58.4.821. [DOI] [PubMed] [Google Scholar]
- Rubin E., Purves D. Segmental organization of sympathetic preganglionic neurons in the mammalian spinal cord. J Comp Neurol. 1980 Jul 1;192(1):163–174. doi: 10.1002/cne.901920111. [DOI] [PubMed] [Google Scholar]
- SPERRY R. W. CHEMOAFFINITY IN THE ORDERLY GROWTH OF NERVE FIBER PATTERNS AND CONNECTIONS. Proc Natl Acad Sci U S A. 1963 Oct;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski A. A. Trophic function of neurons in transplanted neonatal ganglia. Exp Neurol. 1974 Oct;45(1):189–193. doi: 10.1016/0014-4886(74)90111-3. [DOI] [PubMed] [Google Scholar]
- de Olmos J., Hardy H., Heimer L. The afferent connections of the main and the accessory olfactory bulb formations in the rat: an experimental HRP-study. J Comp Neurol. 1978 Sep 15;181(2):213–244. doi: 10.1002/cne.901810202. [DOI] [PubMed] [Google Scholar]