Abstract
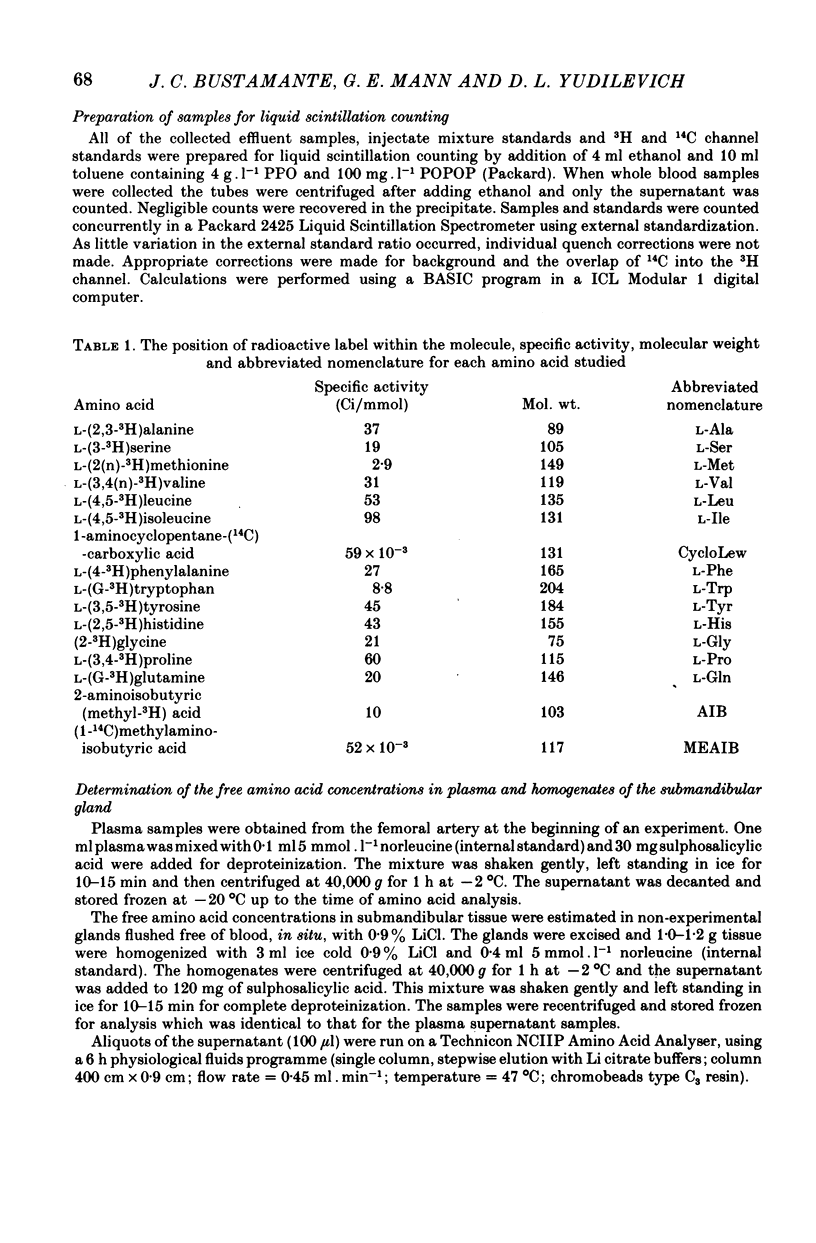
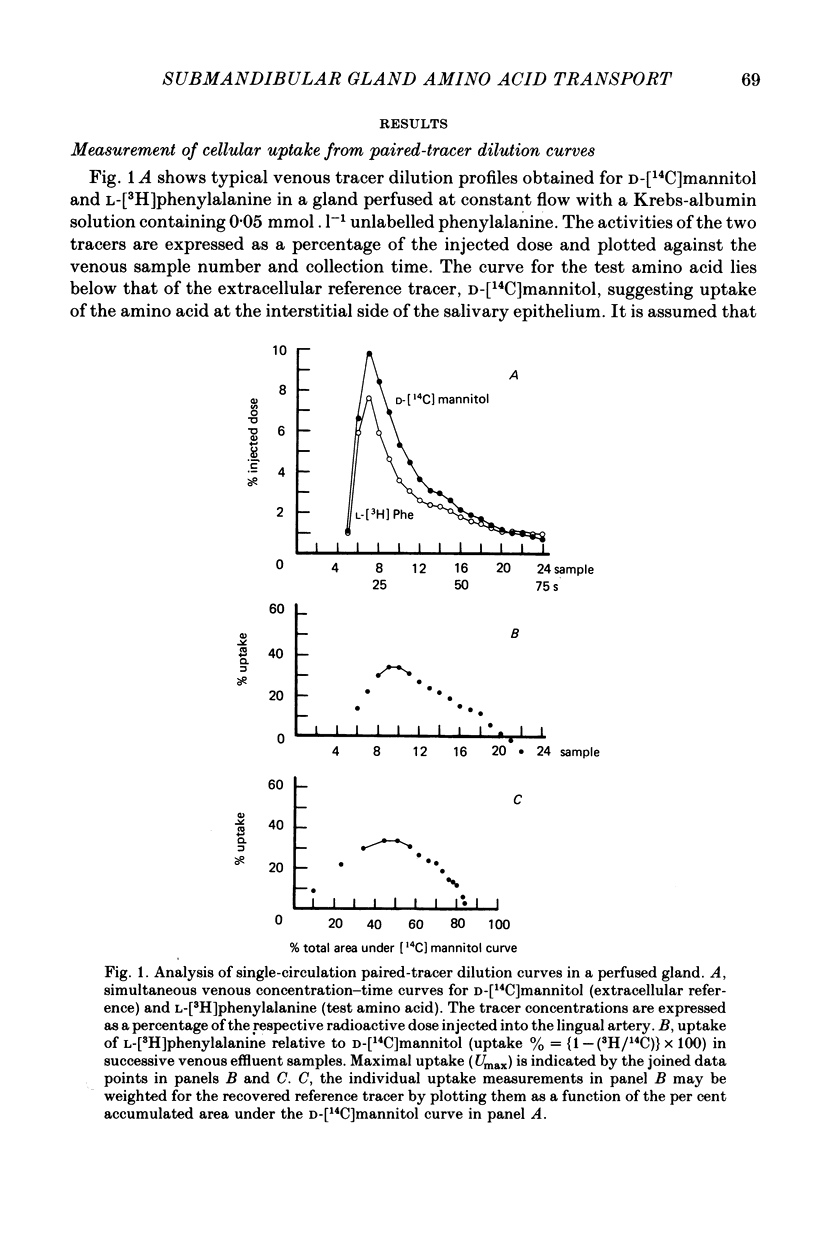
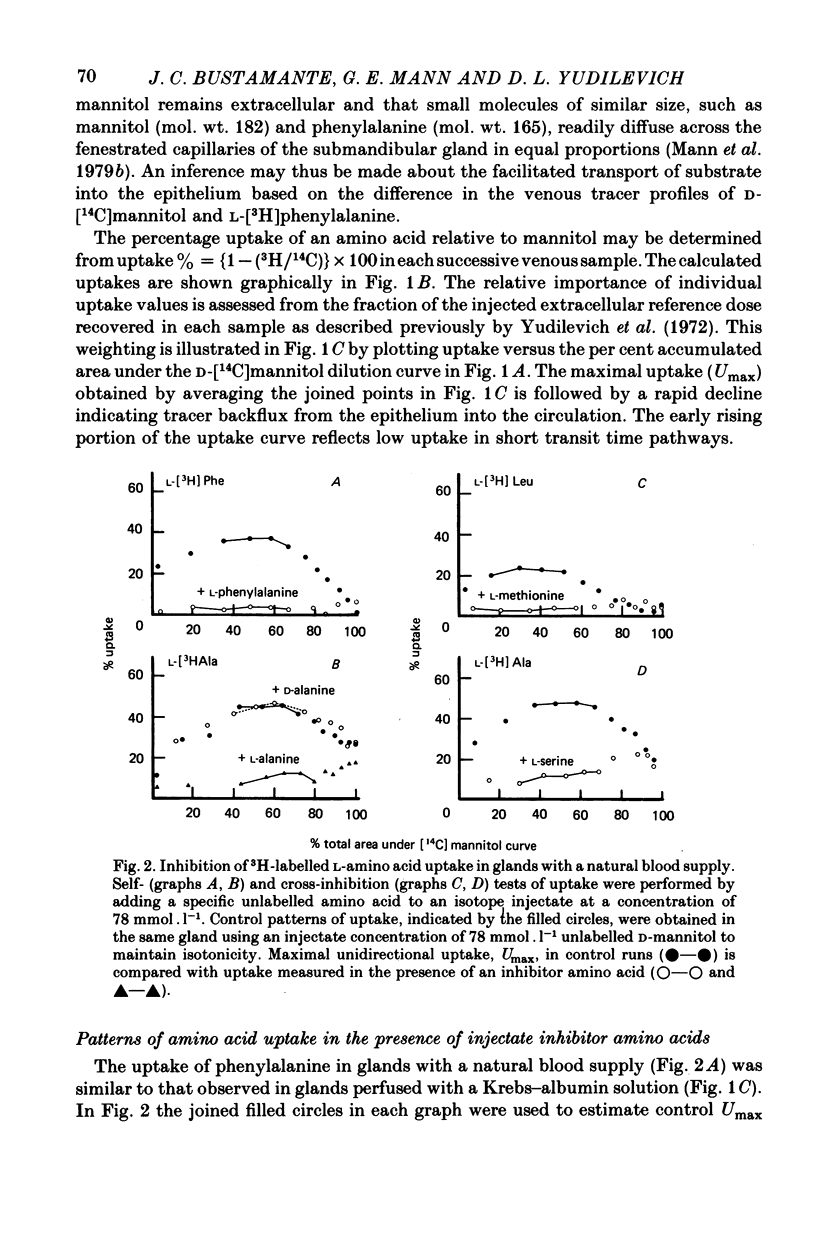
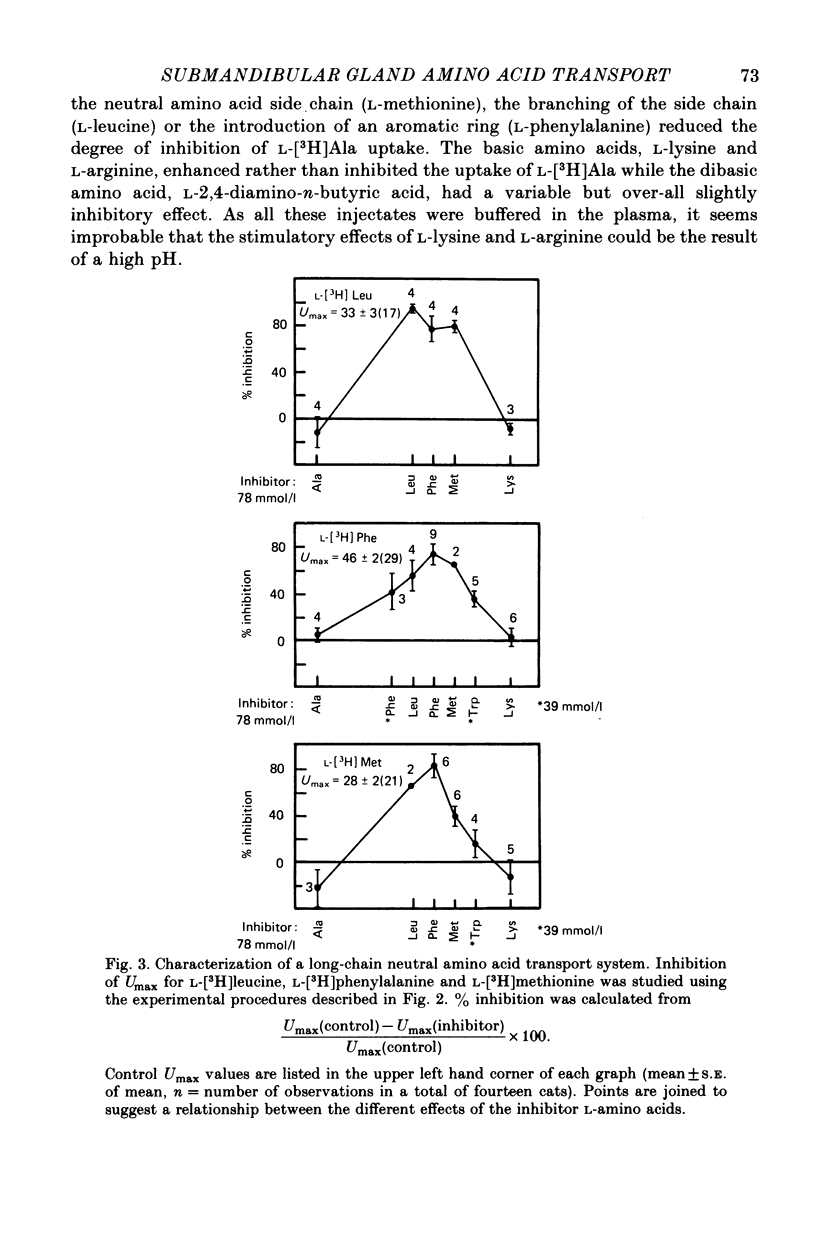
1. Amino acid uptake was measured in resting cat submandibular glands with either a natural blood supply or perfused at constant flow with a Krebs-albumin solution. Following a bolus arterial injection of a 3H-labelled amino acid and D-[14C]mannitol (extracellular reference tracer), the venous effluent was immediately sampled sequentially. The maximal uptake, Umax, from the blood or perfusate was determined from the paired-tracer dilution curves using the expression: uptake % = (1 -- (3H/14C) X 100). 2. In glands with a natural blood supply, Umax values up to 46% were measured for short-chain (serine and alanine) and long-chain (valine, methionine, leucine, isoleucine, 1-amino-cyclopentane cyclopentane carboxylic acid, phenylalanine, tryptophan, tyrosine, histidine and glutamine) neutral amino acids. In contrast, Umax was negligible for amino acids of the imino-glycine group (proline and glycine) and the nonmetabolized amino acids, 2-aminoisobutyric acid (AIB) and methylaminoisobutyric acid (MeAIB). 3. In glands with a natural blood supply addition of an unlabelled amino acid to the tracer injectate reduced Umax for the test acid by up to 80%. The pattern of these interactions suggested the presence of two transport systems for neutral amino acids, one preferring short-chain and the other long-chain amino acids. 4. In glands perfused at constant flow rates with an amino acid-free Krebs-albumin solution high Umax values were measured: L-serine (66%), L-alanine (54%), L-leucine (43%), L-phenylalanine (42%) and L-tyrosine (51%). Only a low uptake was observed for L-proline (8%) and glycine (14%). There was no uptake of methylaminoisobutyric acid which confirms the result obtained in glands with an intact circulation. 5. Saturation of L-phenylalanine influx was observed in perfused glands as the perfusate concentration of unlabelled L-phenylalanine was increased from 0.5 to 20 mmol . 1-1. A Michaelis--Menten analysis based on a single entry system indicated an apparent Km of 6.4 +/- 0.8 mmol . 1-1 and a Vmax of 1719 +/- 94 nmol . min-1g.-1 6. Since the fenestrated capillaries in the salivary gland are readily permeable to the test amino acid and D-mannitol, it is most probable that the amino acid carriers are located in the basolateral side of the epithelium. 7. The use of a paired-tracer dilution technique to measure uptake in a single circulatory passage has enabled a detailed characterization of neutral amino acid transport in the salivary gland and has overcome the limitation of previous studies based on solute transfer from blood to saliva.
Full text
PDF




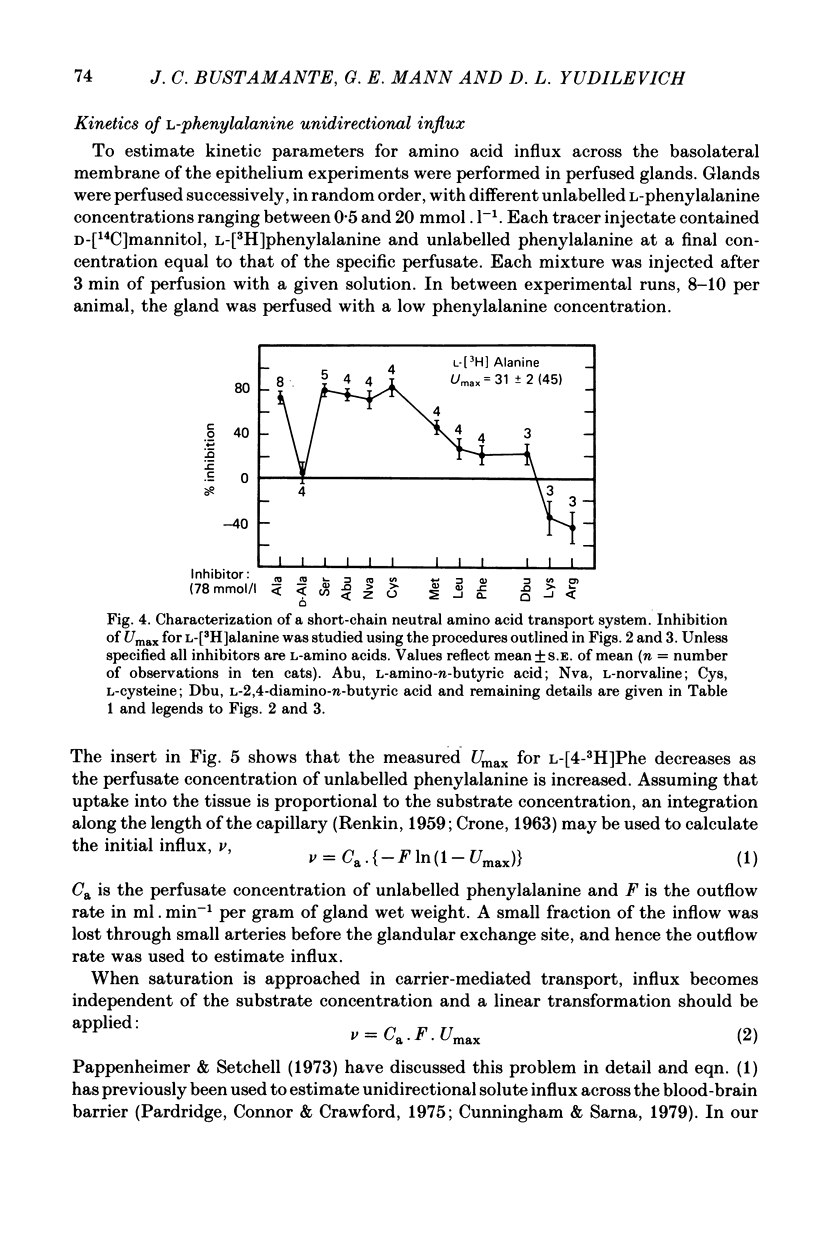




Selected References
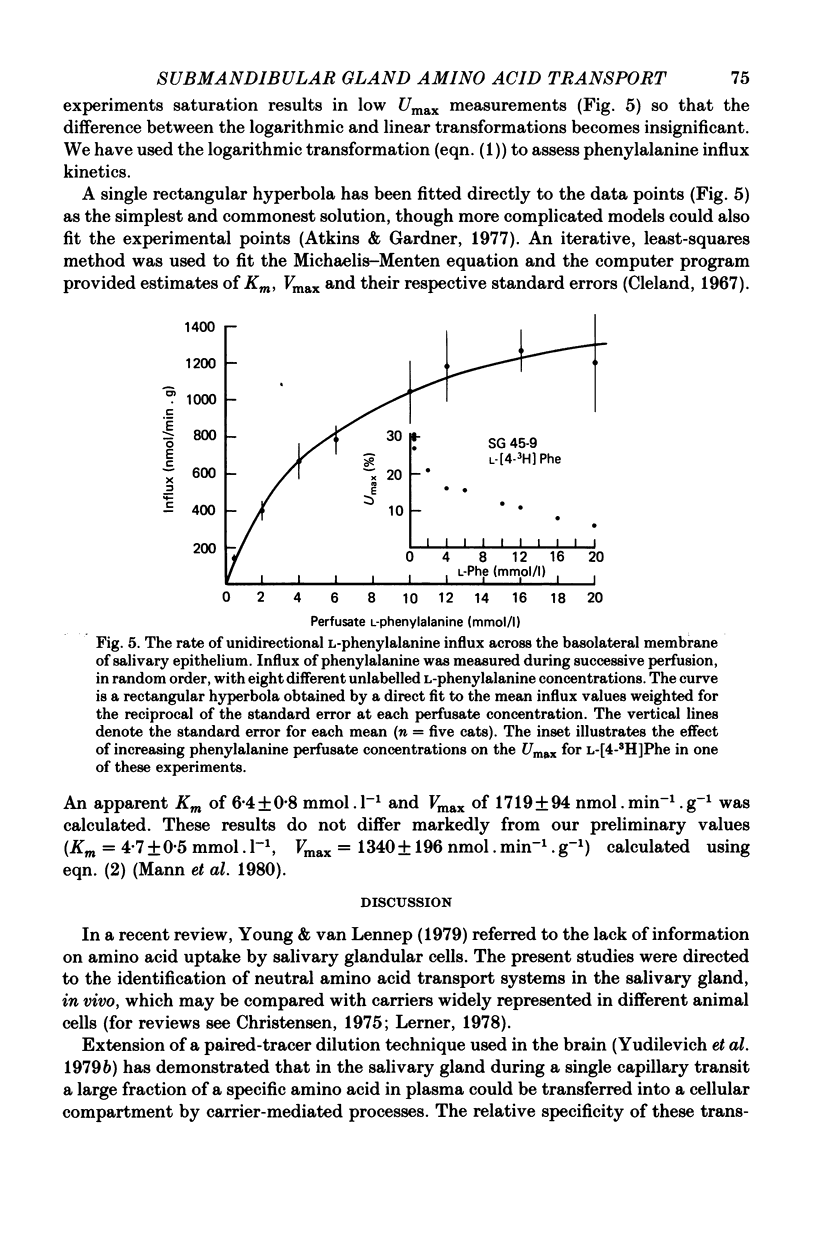
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins G. L., Gardner M. L. The computation of saturable and linear components of intestinal and other transport kinetics. Biochim Biophys Acta. 1977 Jul 4;468(1):127–145. doi: 10.1016/0005-2736(77)90156-0. [DOI] [PubMed] [Google Scholar]
- BURGEN A. S., TERROUX K. G. Influx sites in the duct system of the dog parotid gland. J Physiol. 1962 May;161:399–413. doi: 10.1113/jphysiol.1962.sp006895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baños G., Daniel P. M., Moorhouse S. R., Pratt O. E., Wilson P. A. The influx of amino acids into the heart of the rat. J Physiol. 1978 Jul;280:471–486. doi: 10.1113/jphysiol.1978.sp012395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz A. L., Gilboe D. D., Drewes L. R. Kinetics of unidirectional leucine transport into brain: effects of isoleucine, valine, and anoxia. Am J Physiol. 1975 Mar;228(3):895–900. doi: 10.1152/ajplegacy.1975.228.3.895. [DOI] [PubMed] [Google Scholar]
- Betz A. L., Gilboe D. D., Yudilevich D. L., Drewes L. R. Kinetics of unidirectional glucose transport into the isolated dog brain. Am J Physiol. 1973 Sep;225(3):586–592. doi: 10.1152/ajplegacy.1973.225.3.586. [DOI] [PubMed] [Google Scholar]
- Betz A. L., Goldstein G. W. Polarity of the blood-brain barrier: neutral amino acid transport into isolated brain capillaries. Science. 1978 Oct 13;202(4364):225–227. doi: 10.1126/science.211586. [DOI] [PubMed] [Google Scholar]
- Bustamante J. C., Mann G. E., Yudilevich D. L. An in vivo study of amino acid carriers in the salivary epithelium [proceedings]. J Physiol. 1978 Dec;285:31P–31P. [PubMed] [Google Scholar]
- Bustamante J. C., Mann G. E., Yudilevich D. L. Characterization of neutral amino acid carriers at the basal side of salivary epithelium by paired-tracer single circulation [proceedings]. J Physiol. 1979 Jun;291:26P–28P. [PubMed] [Google Scholar]
- CRONE C. THE PERMEABILITY OF CAPILLARIES IN VARIOUS ORGANS AS DETERMINED BY USE OF THE 'INDICATOR DIFFUSION' METHOD. Acta Physiol Scand. 1963 Aug;58:292–305. doi: 10.1111/j.1748-1716.1963.tb02652.x. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Exploiting amino acid structure to learn about membrane transport. Adv Enzymol Relat Areas Mol Biol. 1979;49:41–101. doi: 10.1002/9780470122945.ch2. [DOI] [PubMed] [Google Scholar]
- Christensen H. N., Handlogten M. E. Interaction between parallel transport systems examined with tryptophan and related amino acids. J Neural Transm Suppl. 1979;(15):1–13. doi: 10.1007/978-3-7091-2243-3_1. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Cunningham V. J., Sarna G. S. Estimation of the kinetic parameters of undirectional transport across the blood-brain barrier. J Neurochem. 1979 Aug;33(2):433–437. doi: 10.1111/j.1471-4159.1979.tb05172.x. [DOI] [PubMed] [Google Scholar]
- Dixon M., Jackson D. M., Richards I. M. The effects of 5-hydroxytryptamine, histamine and acetylcholine on the reactivity of the lung of the anaesthetized dog. J Physiol. 1980 Oct;307:85–96. doi: 10.1113/jphysiol.1980.sp013425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekfors T., Barka T. The effect of isoproterenol on amino acids in the submandibular gland. Lab Invest. 1971 Sep;25(3):287–290. [PubMed] [Google Scholar]
- Guidotti G. G., Borghetti A. F., Gazzola G. C. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978 Dec 15;515(4):329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Christensen H. N., Handlogten M. E. Cysteine as a system-specific substrate for transport system ASC in rat hepatocytes. Biochem Biophys Res Commun. 1979 May 28;88(2):744–751. doi: 10.1016/0006-291x(79)92110-7. [DOI] [PubMed] [Google Scholar]
- Lindsay R. H., Catanzaro O. L., Hanson R. W. Amino acid secretion and metabolism by dog salivary glands. Am J Physiol. 1969 Jul;217(1):233–238. doi: 10.1152/ajplegacy.1969.217.1.233. [DOI] [PubMed] [Google Scholar]
- Lindsay R. H., Catanzaro O. L., Ueha T., Hanson R. W. Site of amino acid transfer in dog parotid glands. Am J Physiol. 1969 Oct;217(4):1025–1029. doi: 10.1152/ajplegacy.1969.217.4.1025. [DOI] [PubMed] [Google Scholar]
- Mann G. E., Smaje L. H., Yudilevich D. L. Permeability of the fenestrated capillaries in the cat submandibular gland to lipid-insoluble molecules. J Physiol. 1979 Dec;297(0):335–354. doi: 10.1113/jphysiol.1979.sp013043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann G. E., Smaje L. H., Yudilevich D. L. Restricted distribution of molecules in the cat salivary gland interstitium [proceedings]. J Physiol. 1979 Apr;289:22P–22P. [PubMed] [Google Scholar]
- Neame K. D. A comparison of the transport systems for amino acids in brain, intestine, kidney and tumour. Prog Brain Res. 1968;29:185–199. doi: 10.1016/S0079-6123(08)64156-4. [DOI] [PubMed] [Google Scholar]
- Pappenheimer J. R., Setchell B. P. Cerebral glucose transport and oxygen consumption in sheep and rabbits. J Physiol. 1973 Sep;233(3):529–551. doi: 10.1113/jphysiol.1973.sp010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Connor J. D., Crawford I. L. Permeability changes in the blood-brain barrier: causes and consequences. CRC Crit Rev Toxicol. 1975 Jan;3(2):159–199. doi: 10.3109/10408447509079857. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Oldendorf W. H. Transport of metabolic substrates through the blood-brain barrier. J Neurochem. 1977 Jan;28(1):5–12. doi: 10.1111/j.1471-4159.1977.tb07702.x. [DOI] [PubMed] [Google Scholar]
- RENKIN E. M. Transport of potassium-42 from blood to tissue in isolated mammalian skeletal muscles. Am J Physiol. 1959 Dec;197:1205–1210. doi: 10.1152/ajplegacy.1959.197.6.1205. [DOI] [PubMed] [Google Scholar]
- TALLAN H. H., MOORE S., STEIN W. H. Studies on the free amino acids and related compounds in the tissues of the cat. J Biol Chem. 1954 Dec;211(2):927–939. [PubMed] [Google Scholar]
- Takada M. Fenestrated venules of the large salivary glands. Anat Rec. 1970 Apr;166(4):605–609. doi: 10.1002/ar.1091660406. [DOI] [PubMed] [Google Scholar]
- Young J. D., Ellory J. C. Substrate specificity of amino acid transport in sheep erythrocytes. Biochem J. 1977 Jan 15;162(1):33–38. doi: 10.1042/bj1620033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudilevich D. L., De Rose N., Sepúlveda F. V. Facilitated transport of amino acids through the blood-brain barrier of the dog studied in a single capillary circulation. Brain Res. 1972 Sep 29;44(2):569–578. doi: 10.1016/0006-8993(72)90319-8. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Eaton B. M. Amino acid carriers at maternal and fetal surfaces of the placenta by single circulation paired-tracer dilution. Kinetics of phenylalanine transport. Biochim Biophys Acta. 1980 Feb 28;596(2):315–319. doi: 10.1016/0005-2736(80)90364-8. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Eaton B. M., Short A. H., Leichtweiss H. P. Glucose carriers at maternal and fetal sides of the trophoblast in guinea pig placenta. Am J Physiol. 1979 Nov;237(5):C205–C212. doi: 10.1152/ajpcell.1979.237.5.C205. [DOI] [PubMed] [Google Scholar]
- van Venrooij W. J., Kuijper-Lenstra A. H., Kramer M. F. Interrelationship between amino acid pools and protein synthesis in the rat submandibular gland. Biochim Biophys Acta. 1973 Jun 23;312(2):392–398. doi: 10.1016/0005-2787(73)90383-3. [DOI] [PubMed] [Google Scholar]


