Abstract
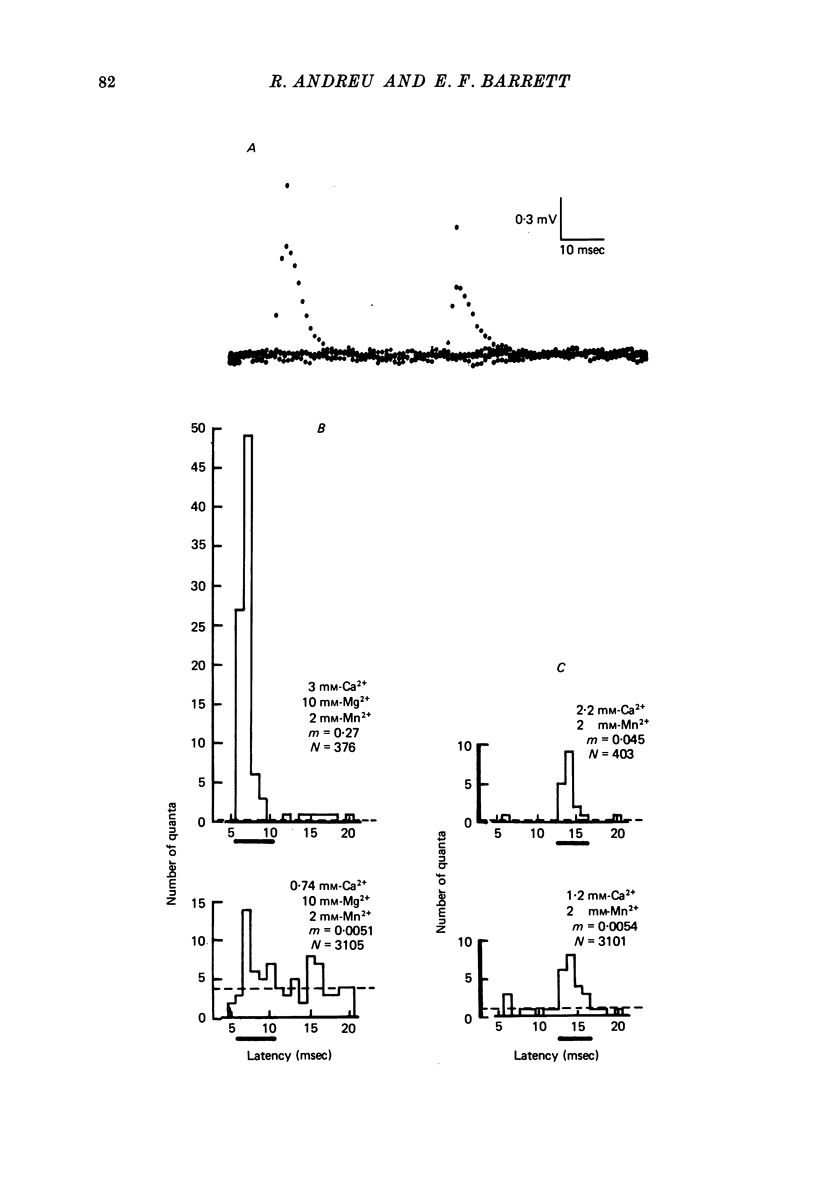
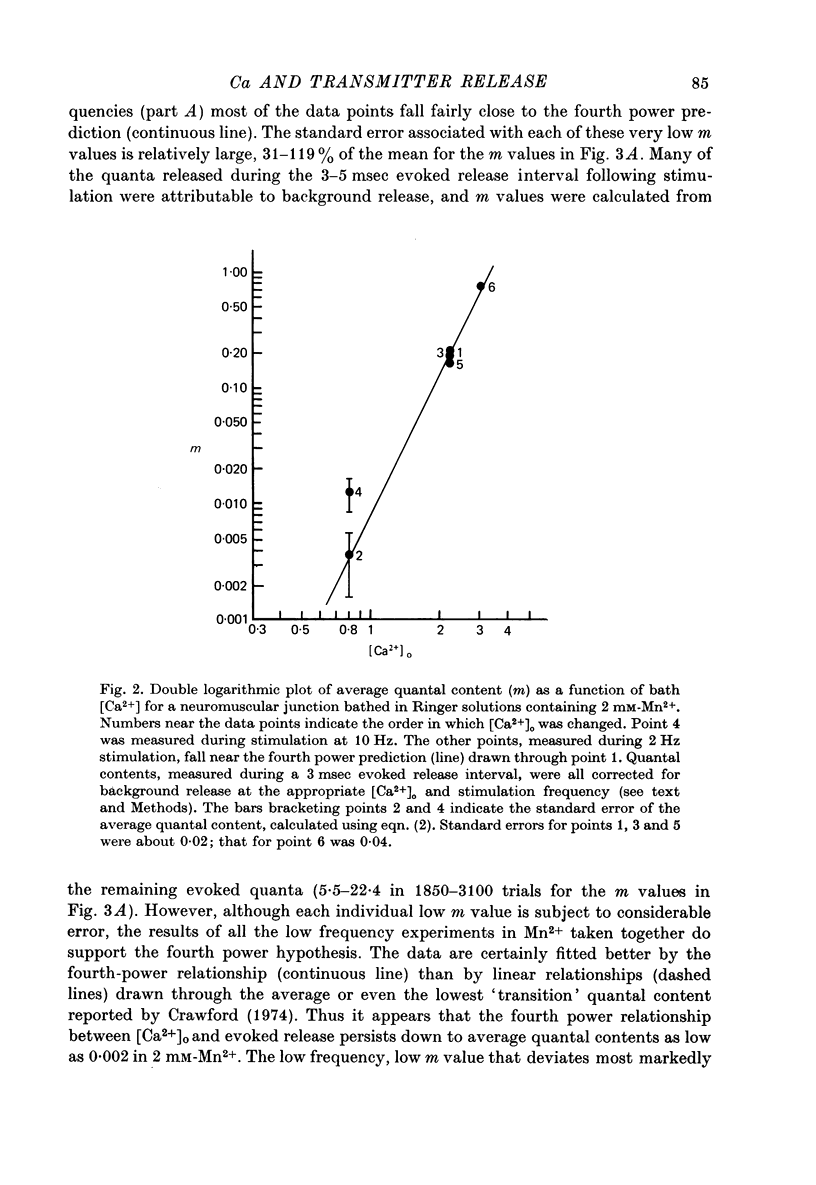
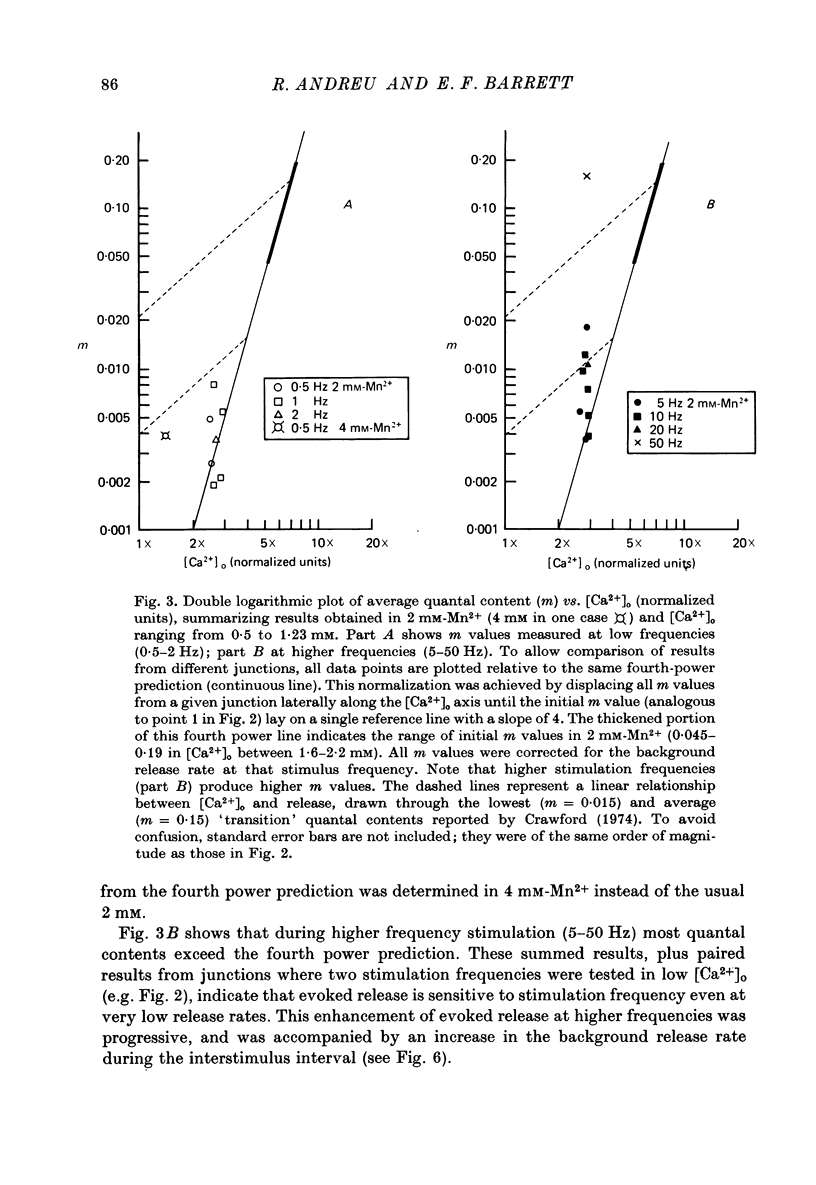
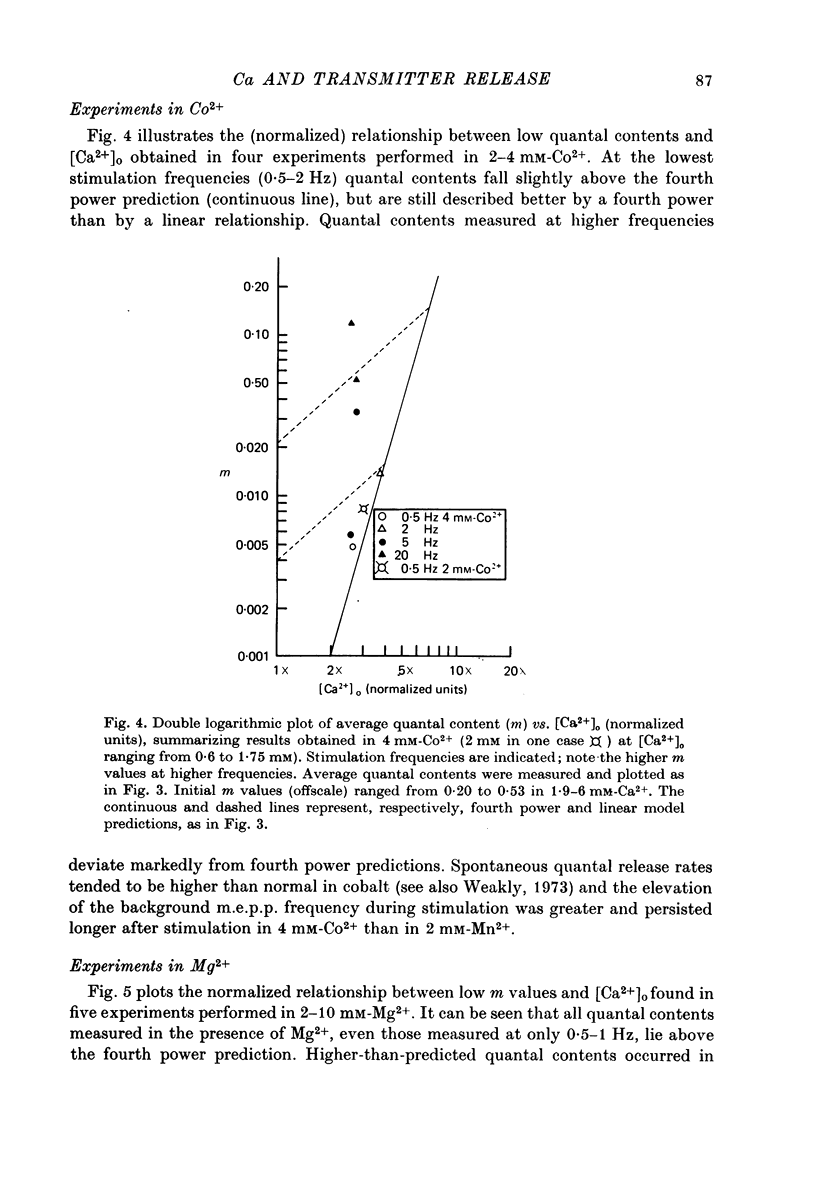
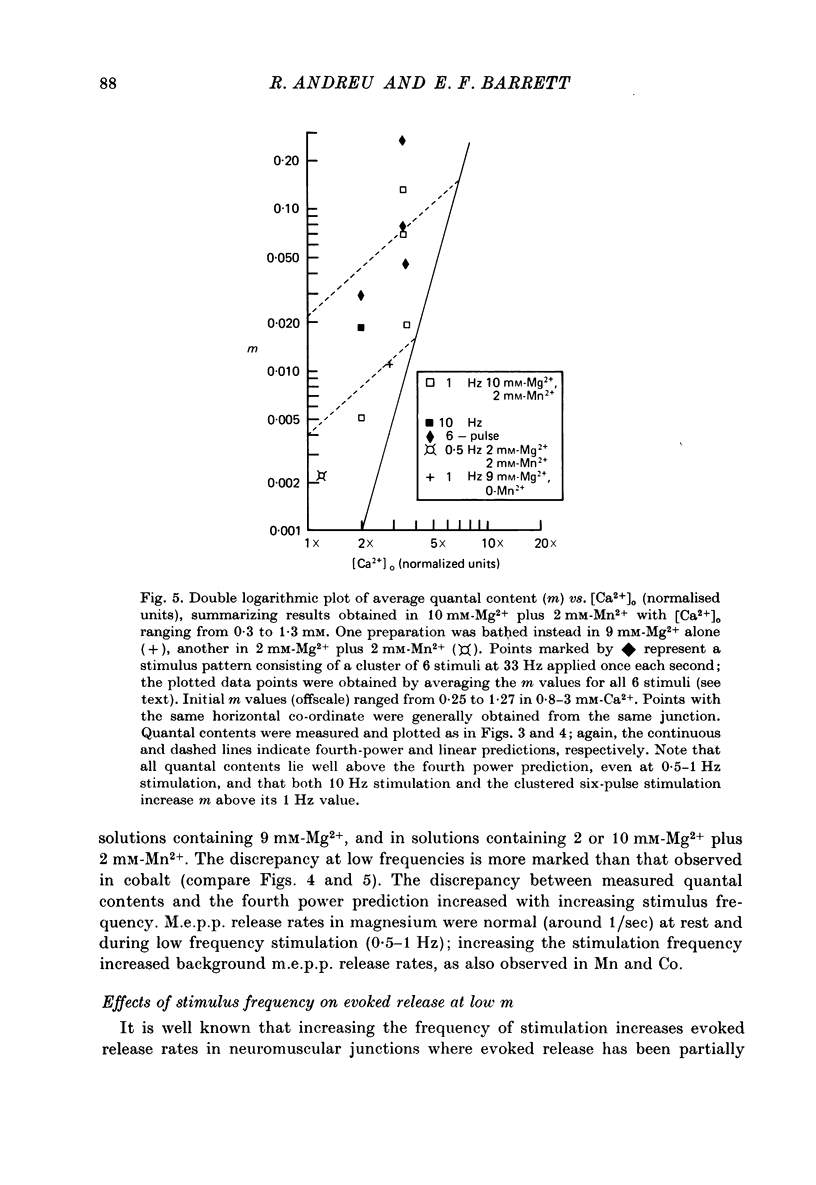
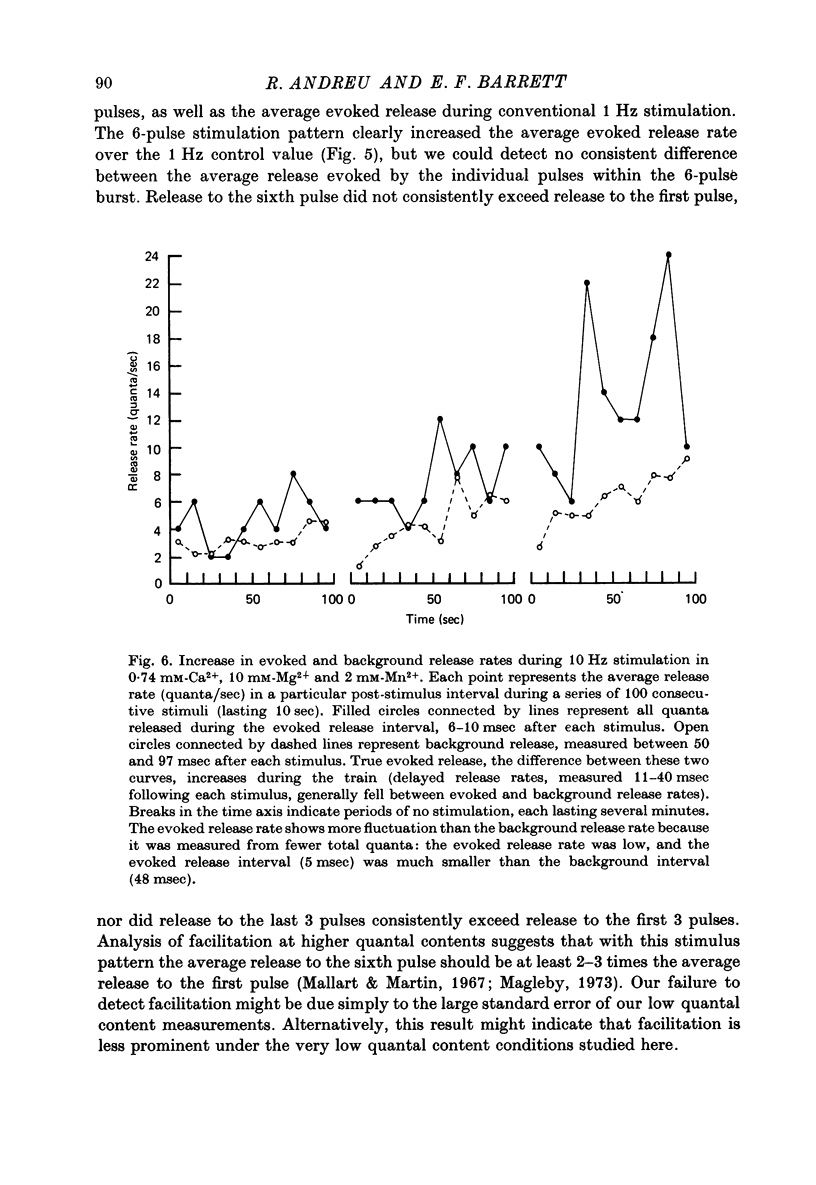
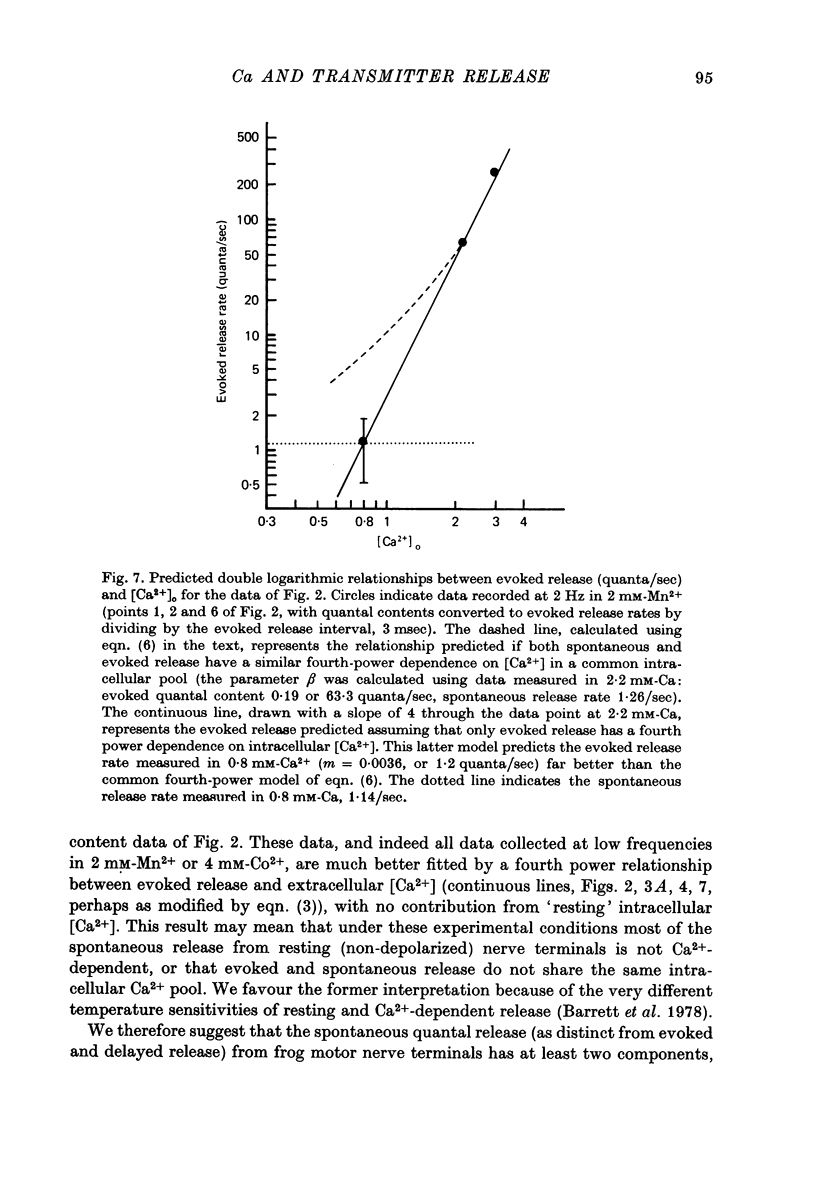
1. The relationship between the rate of evoked transmitter release and the extra-cellular concentration of Ca ions, [Ca2+]o, was studied at surface neuromuscular junctions of the frog cutaneous pectoris muscle. The average quantal content of the end-plate potential was reduced to low levels by reducing [Ca2+]o and adding 2 mM-Mn2+, 45 MM-Co2+ or 10 mM-Mg2+. 2. When the motor nerve was stimulated at a low frequency (0.5--2 Hz) in 2 mM-Mn2+ or 4 mM-Co2+, the average quantal content of evoked release was proportional to the fourth power of [Ca2+]o down to the lowest measurable quantal contents, around 2--4 quanta per 1000 stimuli. Combined with previous studies, this result indicates that evoked transmitter release has a steep, nonlinear dependence on [Ca2+]0 over our orders of magnitude of evoked release. 3. Calculations predict that if evoked and spontaneous release have the same fourth power dependence on intracellular [Ca2+], then the curve relating evoked release and [Ca2+]o should become much less steep as the evoked release rate approaches the spontaneous release rate. Our observation that the relationship between evoked release and [Ca2+]o remains fourth power down to very low release rates suggests that most spontaneous quantal release does not have the same dependence on intracellular [Ca2+], or does not use the same intracellular Ca2 pool, as evoked release. 4. In 2--10 mM-Mg2+, the lowest average quantal contents were markedly higher than the fourth power prediction. This discrepancy may occur either because Mg2+ somehow elevates intracellular [Ca2+], or because Mg2+ is itself a weak activator of transmitter release. 5. Even at very low rates of evoked release, increasing the stimulus frequency to 5--50 Hz caused a progressive increase in both evoked release and the rate of 'background' quantal release during the interstimulus interval. The frequency-dependent enhancement of both evoked and background release was more pronounced in solutions containing 10 mM-Mg2+ than in solutions containing 2 mM-Mn2+.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balnave R. J., Gage P. W. On facilitation of transmitter release at the toad neuromuscular junction. J Physiol. 1974 Jun;239(3):657–675. doi: 10.1113/jphysiol.1974.sp010588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balnave R. J., Gage P. W. The inhibitory effect of manganese on transmitter release at the neuromuscular junction of the toad. Br J Pharmacol. 1973 Feb;47(2):339–352. doi: 10.1111/j.1476-5381.1973.tb08332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Botz D., Chang D. B., Mahaffey D. Temperature-sensitive aspects of evoked and spontaneous transmitter release at the frog neuromuscular junction. J Physiol. 1978 Jun;279:253–273. doi: 10.1113/jphysiol.1978.sp012343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. Quantal independence and uniformity of presynaptic release kinetics at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):665–689. doi: 10.1113/jphysiol.1972.sp010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blioch Z. L., Glagoleva I. M., Liberman E. A., Nenashev V. A. A study of the mechanism of quantal transmitter release at a chemical synapse. J Physiol. 1968 Nov;199(1):11–35. doi: 10.1113/jphysiol.1968.sp008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cooke J. D., Okamoto K., Quastel D. M. The role of calcium in depolarization-secretion coupling at the motor nerve terminal. J Physiol. 1973 Jan;228(2):459–497. doi: 10.1113/jphysiol.1973.sp010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C. The dependence of evoked transmitter release on external calcium ions at very low mean quantal contents. J Physiol. 1974 Jul;240(2):255–278. doi: 10.1113/jphysiol.1974.sp010609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The effect of magnesium on the activity of motor nerve endings. J Physiol. 1954 Jun 28;124(3):553–559. doi: 10.1113/jphysiol.1954.sp005128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Rahamimoff R., Rotshenker S. Quelling of spontaneous transmitter release by nerve impulses in low extracellular calcium solutions. J Physiol. 1978 May;278:491–500. doi: 10.1113/jphysiol.1978.sp012319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Rahamimoff R. The role of calcium ions in tetanic and post-tetanic increase of miniature end-plate potential frequency. J Physiol. 1978 May;278:501–511. doi: 10.1113/jphysiol.1978.sp012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A., Mauro A. Reversibility and mode of action of Black Widow spider venom on the vertebrate neuromuscular junction. J Gen Physiol. 1979 Feb;73(2):245–263. doi: 10.1085/jgp.73.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Fukuda J., Eaton D. C. Membrane currents carried by Ca, Sr, and Ba in barnacle muscle fiber during voltage clamp. J Gen Physiol. 1974 May;63(5):564–578. doi: 10.1085/jgp.63.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut W. P., Longenecker H. B., Jr, Mauro A. Effects of calcium and magnesium on the frequency of miniature end-plate potentials during prolonged tetanization. J Physiol. 1971 Dec;219(1):17–38. doi: 10.1113/jphysiol.1971.sp009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Suppression of transmitter release at the neuromuscular junction. Proc R Soc Lond B Biol Sci. 1977 Apr;196(1125):465–469. doi: 10.1098/rspb.1977.0051. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H., Van der Kloot W. Action of Co and Ni at the frog neuromuscular junction. Nat New Biol. 1973 Sep 12;245(141):52–53. doi: 10.1038/newbio245052a0. [DOI] [PubMed] [Google Scholar]
- Landau E. M., Smolinsky A., Lass Y. Post-tetanic potentiation and facilitation do not share a common calcium-dependent mechanism. Nat New Biol. 1973 Aug 1;244(135):155–157. doi: 10.1038/newbio244155a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Nicholson C. Calcium role in depolarization-secretion coupling: an aequorin study in squid giant synapse. Proc Natl Acad Sci U S A. 1975 Jan;72(1):187–190. doi: 10.1073/pnas.72.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L. The effect of repetitive stimulation on facilitation of transmitter release at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):327–352. doi: 10.1113/jphysiol.1973.sp010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. A quantitative description of tetanic and post-tetanic potentiation of transmitter release at the frog neuromuscular junction. J Physiol. 1975 Feb;245(1):183–208. doi: 10.1113/jphysiol.1975.sp010840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. Augmentation: A process that acts to increase transmitter release at the frog neuromuscular junction. J Physiol. 1976 May;257(2):449–470. doi: 10.1113/jphysiol.1976.sp011378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri U., Rahamimoff R. Neuromuscular transmission: inhibition by manganese ions. Science. 1972 Apr 21;176(4032):308–309. doi: 10.1126/science.176.4032.308. [DOI] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misler S., Hurlbut W. P. Action of black widow spider venom on quantized release of acetylcholine at the frog neuromuscular junction: dependence upon external Mg2+. Proc Natl Acad Sci U S A. 1979 Feb;76(2):991–995. doi: 10.1073/pnas.76.2.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quastel D. M., Hackett J. T., Cooke J. D. Calcium: is it required for transmitter secretion? Science. 1971 Jun 4;172(3987):1034–1036. doi: 10.1126/science.172.3987.1034. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R. A dual effect of calcium ions on neuromuscular facilitation. J Physiol. 1968 Mar;195(2):471–480. doi: 10.1113/jphysiol.1968.sp008468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Yaari Y. Delayed release of transmitter at the frog neuromuscular junction. J Physiol. 1973 Jan;228(1):241–257. doi: 10.1113/jphysiol.1973.sp010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F. A comment on Martin's relation. Biophys J. 1976 Aug;16(8):891–895. doi: 10.1016/S0006-3495(76)85739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weakly J. N. The action of cobalt ions on neuromuscular transmission in the frog. J Physiol. 1973 Nov;234(3):597–612. doi: 10.1113/jphysiol.1973.sp010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younkin S. G. An analysis of the role of calcium in facilitation at the frog neuromuscular junction. J Physiol. 1974 Feb;237(1):1–14. doi: 10.1113/jphysiol.1974.sp010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Transmitter release during repetitive stimulation: selective changes produced by Sr2+ and Ba2+. Science. 1977 Jul 1;197(4298):67–69. doi: 10.1126/science.17160. [DOI] [PubMed] [Google Scholar]


