Abstract
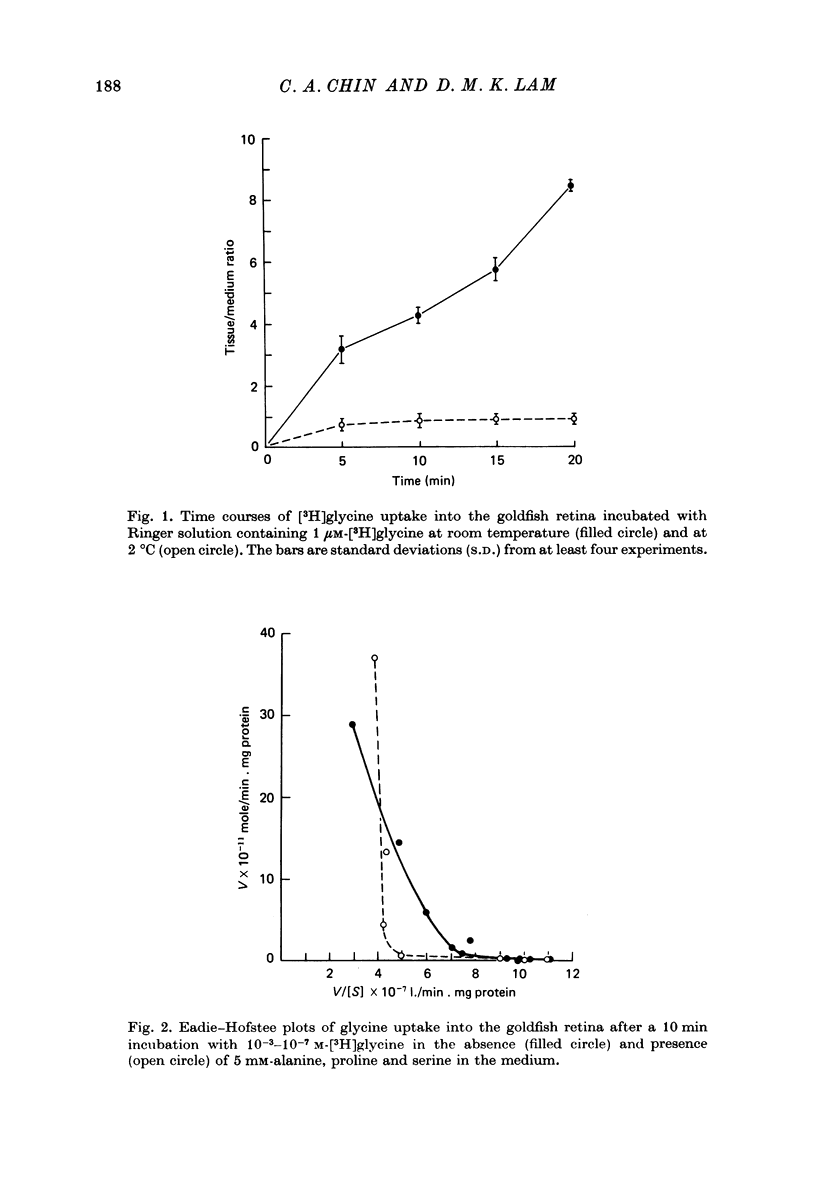
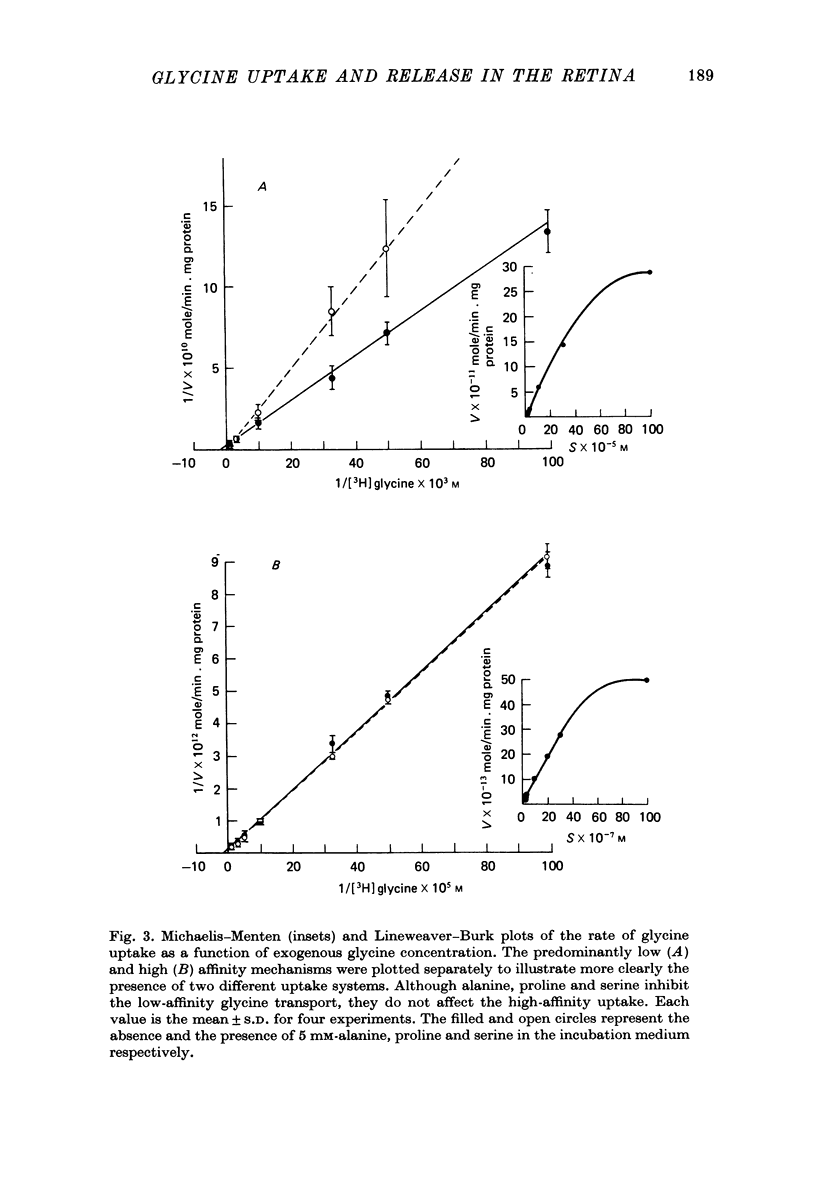
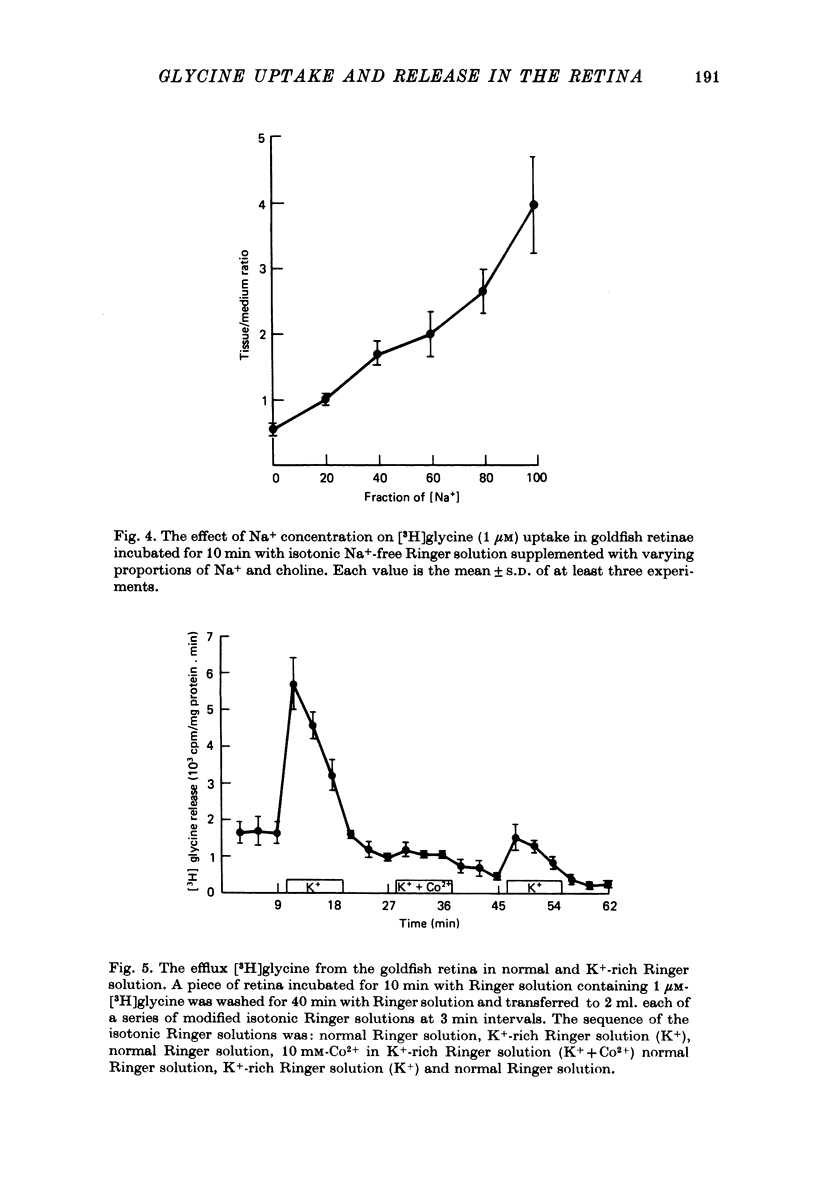
1. In the goldfish retina, uptake of exogenous [3H]glycine follows Michaelis--Menten kinetics with increasing concentrations of glycine. This uptake can be explained kinetically by the presence of two independent affinity systems: a 'high-affinity' mechanism with an apparent Km(H) of 8.1 microM and a Vmax(H) of 9.12 p-moles/min. mg protein, and a 'low-affinity' mechanism with an apparent Km(L) of 0.63 mM and a Vmax(L) of 430 p-mole/min . mg protein. 2. The high-affinity mechanism, and probably also the low-affinity mechanism, is temperature- and Na+-dependent. 3. The low-affinity mechanism for glycine uptake is not affected by 5 mM-isoleucine, methionine and valine in the medium. However, it is inhibited more than 90% by 5 mM-alanine, proline and serine in the medium. This result indicates that the low-affinity transport for glycine may go through system A of the neutral amino acid transport system which is present in most tissues to transport glycine and certain neutral amino acids for metabolic purposes. 4. The high-affinity mechanism for glycine uptake is, however, not affected by the presence of up to 100-fold excess of all amino acids examined. 5. Autoradiographic studies show that at least one type of amacrine cell and one type of probable interplexiform cell take up [3H]glycine both in the presence and absence of 5 mM-alanaine, proline and serine, indicating that these neurones possess the high-affinity mechanism for glycine uptake. 6. [3H]Glycine accumulated in the retina can be released by increasing the external K+ concentration. This release is probably Ca2+-dependent since it is blocked by 10 mM-Co2+ in the medium. Additionally, autoradiographic studies show that [3H]glycine taken up by the glycine-accumulating neurones can also be released by Ca2+-dependent, K+-depolarization of the retina.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames A., 3rd, Pollen D. A. Neurotransmission in central nervous tissue: a study of isolated rabbit retina. J Neurophysiol. 1969 May;32(3):424–442. doi: 10.1152/jn.1969.32.3.424. [DOI] [PubMed] [Google Scholar]
- Baughman R. W., Bader C. R. Biochemical characterization and cellular localization of the cholinergic system in the chicken retina. Brain Res. 1977 Dec 23;138(3):469–485. doi: 10.1016/0006-8993(77)90684-9. [DOI] [PubMed] [Google Scholar]
- Bruun A., Ehinger B. Uptake of the putative neurotransmitter, glycine, into the rabbit retina. Invest Ophthalmol. 1972 Apr;11(4):191–198. [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: changes in centre surround receptive fields. J Physiol. 1978 Mar;276:299–310. doi: 10.1113/jphysiol.1978.sp012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. H., Daw N. W., Wyatt H. J. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J Physiol. 1978 Mar;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ehinger B. The interplexiform cell system. I. Synapses of the dopaminergic neurons of the goldfish retina. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):7–26. doi: 10.1098/rspb.1978.0030. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Lindberg-Bauer B. Light-evoked release of glycine from cat and rabbit retina. Brain Res. 1976 Sep 3;113(3):535–549. doi: 10.1016/0006-8993(76)90055-x. [DOI] [PubMed] [Google Scholar]
- Inui Y., Christensen H. N. Discrimination of single transport systems. The Na plus-sensitive transport of neutral amino acids in the Ehrlich cell. J Gen Physiol. 1966 Sep;50(1):203–224. doi: 10.1085/jgp.50.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Bloom F. E. Studies of the uptake of 3 H-gaba and ( 3 H)glycine in slices and homogenates of rat brain and spinal cord by electron microscopic autoradiography. Brain Res. 1972 Jun 8;41(1):131–143. doi: 10.1016/0006-8993(72)90621-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lam D. M., Lasater E. M., Naka K. I. gamma-Aminobutyric acid: a neurotransmitter candidate for cone horizontal cells of the catfish retina. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6310–6313. doi: 10.1073/pnas.75.12.6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Steinman L. The uptake of ( - 3 H) aminobutyric acid in the goldfish retina. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2777–2781. doi: 10.1073/pnas.68.11.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Su Y. Y., Swain L., Marc R. E., Brandon C., Wu J. Y. Immunocytochemical localisation of L-glutamic acid decarboxylase in the goldfish retina. Nature. 1979 Apr 5;278(5704):565–567. doi: 10.1038/278565a0. [DOI] [PubMed] [Google Scholar]
- Lam D. M. Synaptic chemistry of identified cells in the vertebrate retina. Cold Spring Harb Symp Quant Biol. 1976;40:571–579. doi: 10.1101/sqb.1976.040.01.053. [DOI] [PubMed] [Google Scholar]
- Lam D. M. The biosynthesis and content of gamma-aminobutyric acid in the goldifsh retina. J Cell Biol. 1972 Aug;54(2):225–231. doi: 10.1083/jcb.54.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc R. E., Stell W. K., Bok D., Lam D. M. GABA-ergic pathways in the goldfish retina. J Comp Neurol. 1978 Nov 15;182(2):221–244. doi: 10.1002/cne.901820204. [DOI] [PubMed] [Google Scholar]
- McAdoo D. J., Iliffe T. M., Price C. H., Novak R. A. Specific glycine uptake by identified neurons of Aplysia californica. II. Biochemistry. Brain Res. 1978 Oct 6;154(1):41–51. doi: 10.1016/0006-8993(78)91049-1. [DOI] [PubMed] [Google Scholar]
- Miller R. F., Dacheux R. F., Frumkes T. E. Amacrine cells in Necturus retina: evidence for independent gamma-aminobutyric acid- and glycine-releasing neurons. Science. 1977 Nov 18;198(4318):748–750. doi: 10.1126/science.910159. [DOI] [PubMed] [Google Scholar]
- Neal M. J., Gilroy J. High-affinity choline transport in the isolated retina. Brain Res. 1975 Aug 15;93(3):548–551. doi: 10.1016/0006-8993(75)90197-3. [DOI] [PubMed] [Google Scholar]
- OXENDER D. L., CHRISTENSEN H. N. DISTINCT MEDIATING SYSTEMS FOR THE TRANSPORT OF NEUTRAL AMINO ACIDS BY THE EHRLICH CELL. J Biol Chem. 1963 Nov;238:3686–3699. [PubMed] [Google Scholar]
- Price C. H., Coggeshall R. E., McAdoo D. Specific glycine uptake by identified neurons of Aplysia californica. I. Autoradiography. Brain Res. 1978 Oct 6;154(1):25–40. doi: 10.1016/0006-8993(78)91048-x. [DOI] [PubMed] [Google Scholar]
- Price D. L., Stocks A., Griffin J. W., Young A., Peck K. Glycine-specific synapses in rat spinal cord. Identification by electron microscope autoradiography. J Cell Biol. 1976 Feb;68(2):389–395. doi: 10.1083/jcb.68.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy P. V., Lam D. M. The uptake and release of [3H]dopamine in the goldfish retina. J Neurochem. 1979 Apr;32(4):1269–1277. doi: 10.1111/j.1471-4159.1979.tb11054.x. [DOI] [PubMed] [Google Scholar]
- Sepúlveda F. V., Smith M. W. Discrimination between different entry mechanisms for neutral amino acids in rabbit ileal mucosa. J Physiol. 1978 Sep;282:73–90. doi: 10.1113/jphysiol.1978.sp012449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voaden M. J. Light and the spontaneous efflux of radioactive glycine from the frog retina. Exp Eye Res. 1974 May;18(5):467–475. doi: 10.1016/0014-4835(74)90083-9. [DOI] [PubMed] [Google Scholar]
- Voaden M. J., Marshall J., Murani N. The uptake of [3H]gamma-amino butyric acid and [3H]glycine by the isolated retina of the frog. Brain Res. 1974 Feb 15;67(1):115–132. doi: 10.1016/0006-8993(74)90302-3. [DOI] [PubMed] [Google Scholar]