Abstract
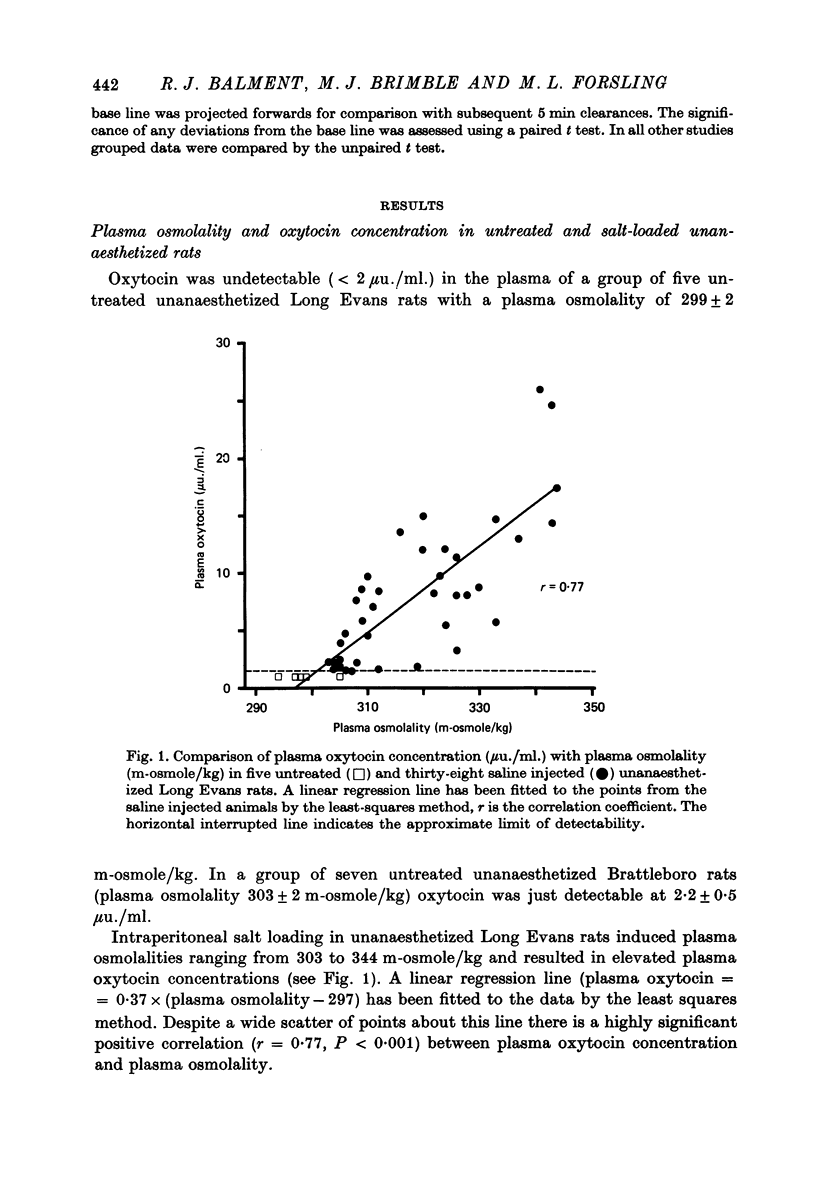
1. The present study investigates the nature and magnitude of the renal response to plasma levels of oxytocin which might be induced by salt loading. 2. Increased plasma osmolality induced by loading with NaCl is an effective stimulus for oxytocin release in the unanaesthetized male rat. Plasma oxytocin concentration was positively correlated (r = 0-.77) with plasma osmolality. Plasma oxytocin (muu./ml.) = 0.37 x (plasma osmolality (m-osmole/kg) -297). 3. In anaesthetized Long Evans rats intra-atrial administration of oxytocin at rates of 0.05 and 0.15 m-u./ml. produced plasma hormone concentrations (5 +/- 1 and 16 +/- 2 mum./ml. respectively) within the range induced by salt loading. 4. Oxytocin administration at 0.15 and 1.5 m-u./min in Long Evans rats produced dose-related increases in urine flow and Na+ and Cl- excretion. Renal responses to 0.05 m-u. oxytocin/min were equivocal. 5. Oxytocin administration at 0.15 m-u./min was ineffective in Brattleboro rats but 1.5 m-u./min led to increased Na+ and Cl- excretion and a reduction in urine flow. 6. Plasma oxytocin levels similar to those induced by severe dehydration or salt loading are effective in increasing renal Na+ and Cl- excretion and urine flow. These effects on water and electrolyte excretion appear to be independent of each other and both may be modified by the presence or absence of vasopressin. 7. This study provides no evidence for a major role for oxytocin in the day to day regulation of salt or water balance under conditions of normal hydration in the male rat.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKS F. P., PICKFORD M. The effect of posterior pituitary hormones on the excretion of electrolytes, in dogs. J Physiol. 1958 Aug 6;142(3):468–493. doi: 10.1113/jphysiol.1958.sp006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balment R. J., Brimble M. J., Forsling M. L. Effect of oxytocin on renal sodium and chloride excretion in Long Evans and Brattleboro rats [proceedings]. J Physiol. 1979 Nov;296:89P–89P. [PubMed] [Google Scholar]
- Balment R. J., Henderson I. W., Oliver J. A. The effects of vasopressin on pituitary oxytocin content and plasma renin activity in rats with hypothalamic diabetes insipidus (Brattleboro strain). Gen Comp Endocrinol. 1975 Aug;26(4):468–477. doi: 10.1016/0016-6480(75)90169-0. [DOI] [PubMed] [Google Scholar]
- Balment R. J., Jones I. C., Henderson I. W., Oliver J. A. Effects of adrenalectomy and hypophysectomy on water and electrolyte metabolism in male and female rats with inherited hypothalamic diabetes insipidus (Brattleboro strain). J Endocrinol. 1976 Nov;71(2):193–217. doi: 10.1677/joe.0.0710193. [DOI] [PubMed] [Google Scholar]
- Brimble M. J., Dyball R. E. Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J Physiol. 1977 Sep;271(1):253–271. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimble M. J., Dyball R. E., Forsling M. L. Oxytocin release following osmotic activation of oxytocin neurones in the paraventricular and supraoptic nuclei. J Physiol. 1978 May;278:69–78. doi: 10.1113/jphysiol.1978.sp012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butron A. M., Illingworth D. V., Challis J. R., McNeilly A. S. Placental transfer of oxytocin in the guinea-pig and its release during parturition. J Endocrinol. 1974 Mar;60(3):499–506. doi: 10.1677/joe.0.0600499. [DOI] [PubMed] [Google Scholar]
- Chan W. Y. An investigation of the natriuretic, antidiuretic and oxytocic actions of neurohypophysial hormones and related peptides: delineation of separate mechanisms of action and assessment of molecular requirements. J Pharmacol Exp Ther. 1976 Mar;196(3):746–757. [PubMed] [Google Scholar]
- Chan W. Y. Effects of neurohypophysial hormones and their deamino analogues on renal excretion of Na, K and water in rats. Endocrinology. 1965 Dec;77(6):1097–1104. doi: 10.1210/endo-77-6-1097. [DOI] [PubMed] [Google Scholar]
- Chan W. Y., Sawyer W. H. Intracranial action of oxytocin on sodium excretion by conscious dogs. Proc Soc Exp Biol Med. 1968 Jan;127(1):267–270. doi: 10.3181/00379727-127-32669. [DOI] [PubMed] [Google Scholar]
- Chard T., Boyd N. R., Forsling M. L., McNeilly A. S., Landon J. The development of a radioimmunoassay for oxytocin: the extraction of oxytocin from plasma, and its measurement during parturition in human and goat blood. J Endocrinol. 1970 Oct;48(2):223–234. doi: 10.1677/joe.0.0480223. [DOI] [PubMed] [Google Scholar]
- Dogterom J., Van Wimersma Greidanus T. B., Swabb D. F. Evidence for the release of vasopressin and oxytocin into cerebrospinal fluid: measurements in plasma and CSF of intact and hypophysectomized rats. Neuroendocrinology. 1977;24(2):108–118. doi: 10.1159/000122702. [DOI] [PubMed] [Google Scholar]
- Dunn F. L., Brennan T. J., Nelson A. E., Robertson G. L. The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest. 1973 Dec;52(12):3212–3219. doi: 10.1172/JCI107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian M., Forsling M. L., Jones J. J., Lee J. The release, clearance and plasma protein binding of oxytocin in the anaesthetized rat. J Endocrinol. 1969 Feb;43(2):175–189. doi: 10.1677/joe.0.0430175. [DOI] [PubMed] [Google Scholar]
- Fraser A. M. The action of the oxytocic hormone of the pituitary gland on urine secretion. J Physiol. 1942 Aug 18;101(2):236–251. doi: 10.1113/jphysiol.1942.sp003978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazis D. Plasma half-lives of vasopressin and oxytocin analogs after iv injection in rats. Proc Soc Exp Biol Med. 1978 Sep;158(4):663–665. doi: 10.3181/00379727-158-40269. [DOI] [PubMed] [Google Scholar]
- JACOBSON H. N., KELLOGG R. H. Isotonic NaCl diuresis in rats: antidiuresis and chloruresis produced by posterior pituitary extracts. Am J Physiol. 1956 Feb;184(2):376–389. doi: 10.1152/ajplegacy.1956.184.2.376. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Pickering B. T. Comparison of the effects of water deprivation and sodium chloride imbibition on the hormone content of the neurohypophysis of the rat. J Physiol. 1969 Aug;203(2):449–458. doi: 10.1113/jphysiol.1969.sp008874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karonen S. L., Mörsky P., Siren M., Seuderling U. An enzymatic solid-phase method for trace iodination of proteins and peptides with 125-iodine. Anal Biochem. 1975 Jul;67(1):1–10. doi: 10.1016/0003-2697(75)90266-3. [DOI] [PubMed] [Google Scholar]
- Möhring B., Möhring J., Dauda G., Haack D. Potassium deficiency in rats with hereditary diabetes insipidus. Am J Physiol. 1974 Oct;227(4):916–920. doi: 10.1152/ajplegacy.1974.227.4.916. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B., Dyball R. E. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B Biol Sci. 1977 Apr;196(1125):367–384. doi: 10.1098/rspb.1977.0046. [DOI] [PubMed] [Google Scholar]
- Robertson G. L., Shelton R. L., Athar S. The osmoregulation of vasopressin. Kidney Int. 1976 Jul;10(1):25–37. doi: 10.1038/ki.1976.76. [DOI] [PubMed] [Google Scholar]
- Sawyer W. H., Valtin H. Antidiuretic responses of rats with hereditary hypothalamic diabetes insipidus to vasopressin, oxytocin and nicotine. Endocrinology. 1967 Jan;80(1):207–210. doi: 10.1210/endo-80-1-207. [DOI] [PubMed] [Google Scholar]
- Sedláková E., Lichardus B., Cort J. H. Plasma saluretic activity: its nature and relation to oxytocin analogs. Science. 1969 May 2;164(3879):580–582. doi: 10.1126/science.164.3879.580. [DOI] [PubMed] [Google Scholar]
- Swaab D. F., Nijveldt F., Pool C. W. Distribution of oxytocin and vasopressin in the rat supraoptic and paraventricular nucleus. J Endocrinol. 1975 Dec;67(3):461–462. doi: 10.1677/joe.0.0670461. [DOI] [PubMed] [Google Scholar]
- Valtin H. Hereditary hypothalamic diabetes insipidus in rats (Brattleboro strain). A useful experimental model. Am J Med. 1967 May;42(5):814–827. doi: 10.1016/0002-9343(67)90098-8. [DOI] [PubMed] [Google Scholar]


