Abstract
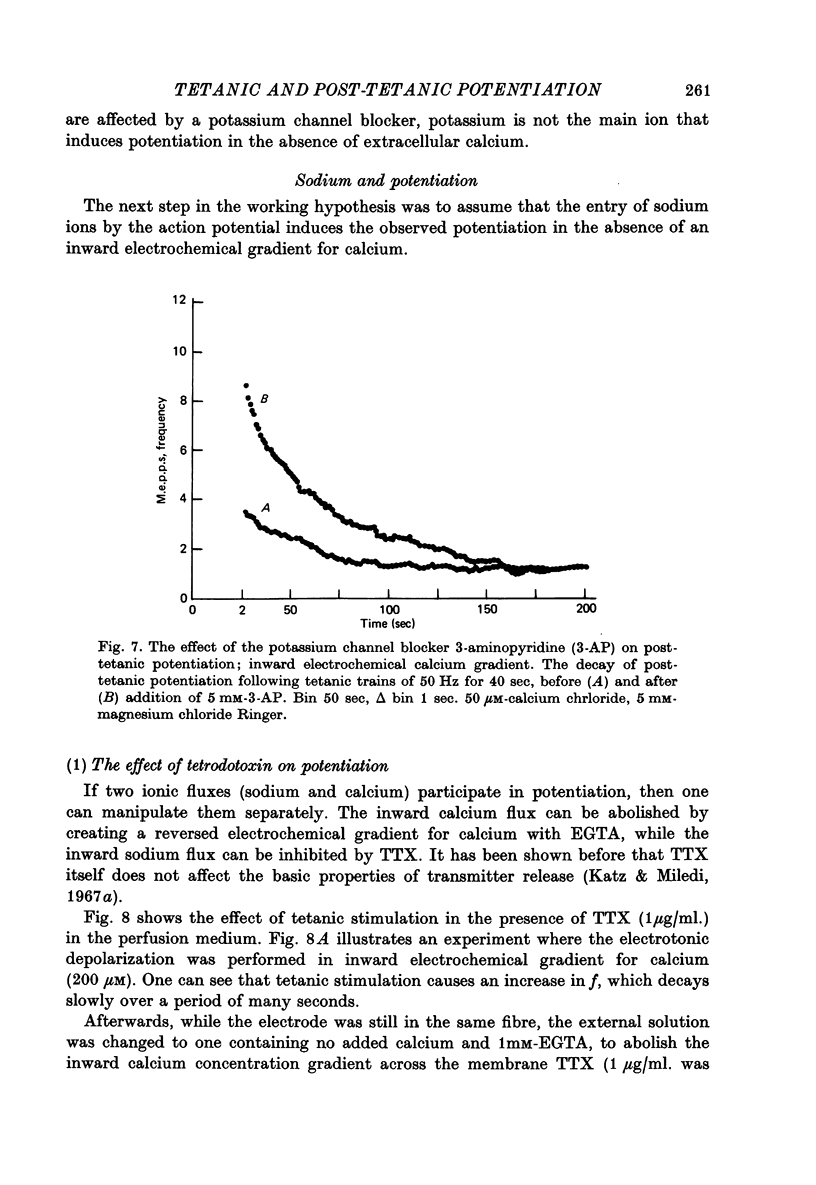
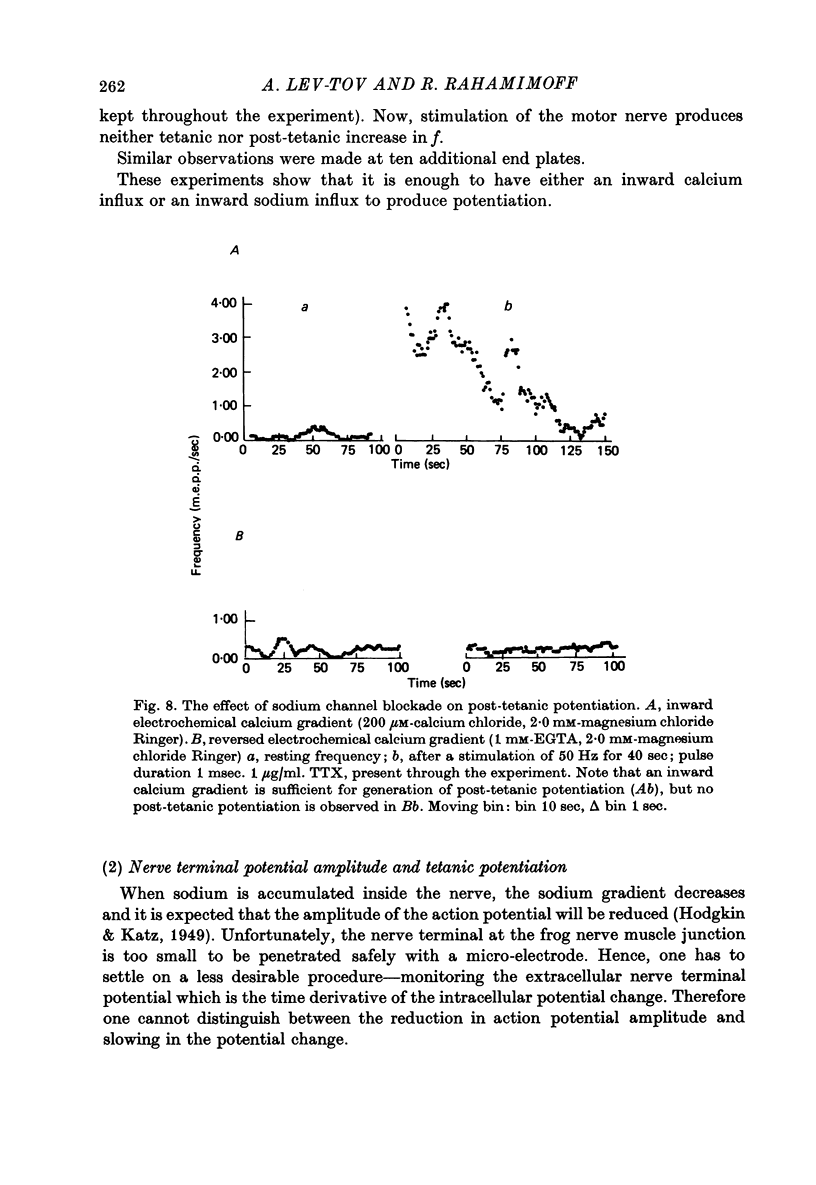
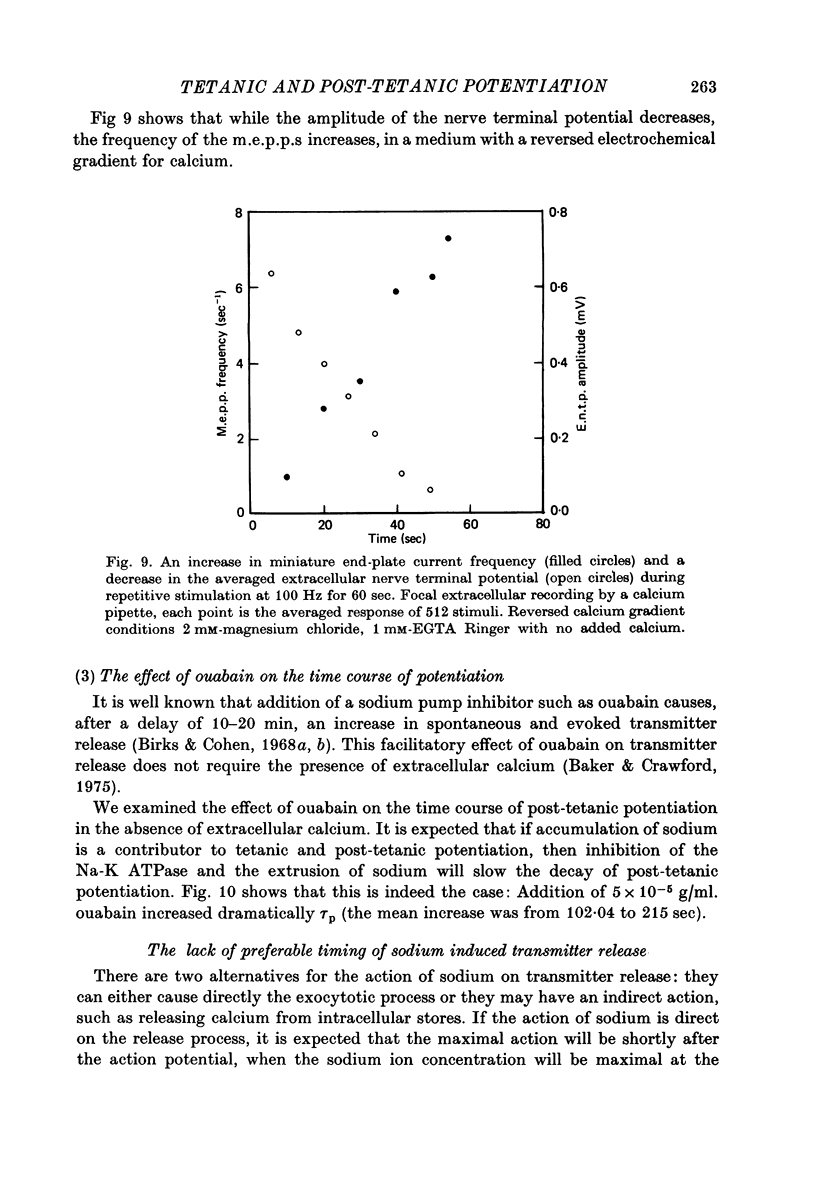
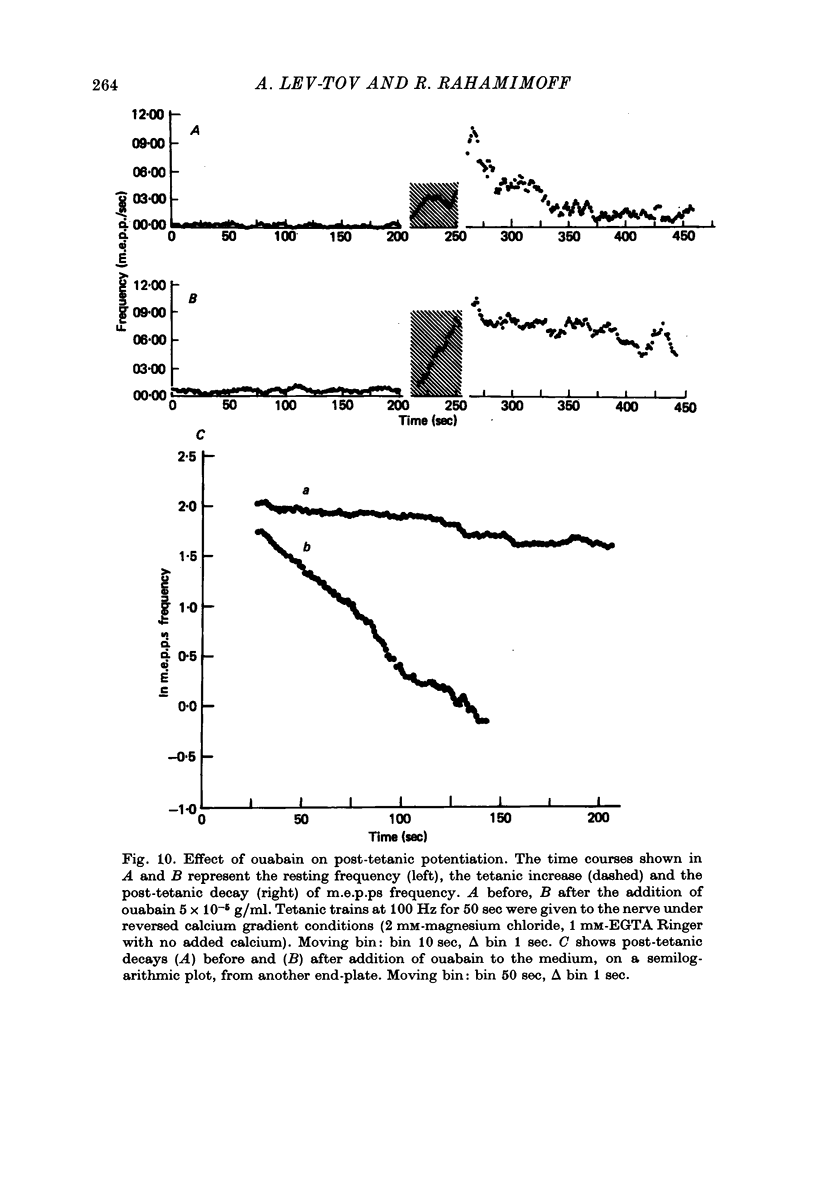
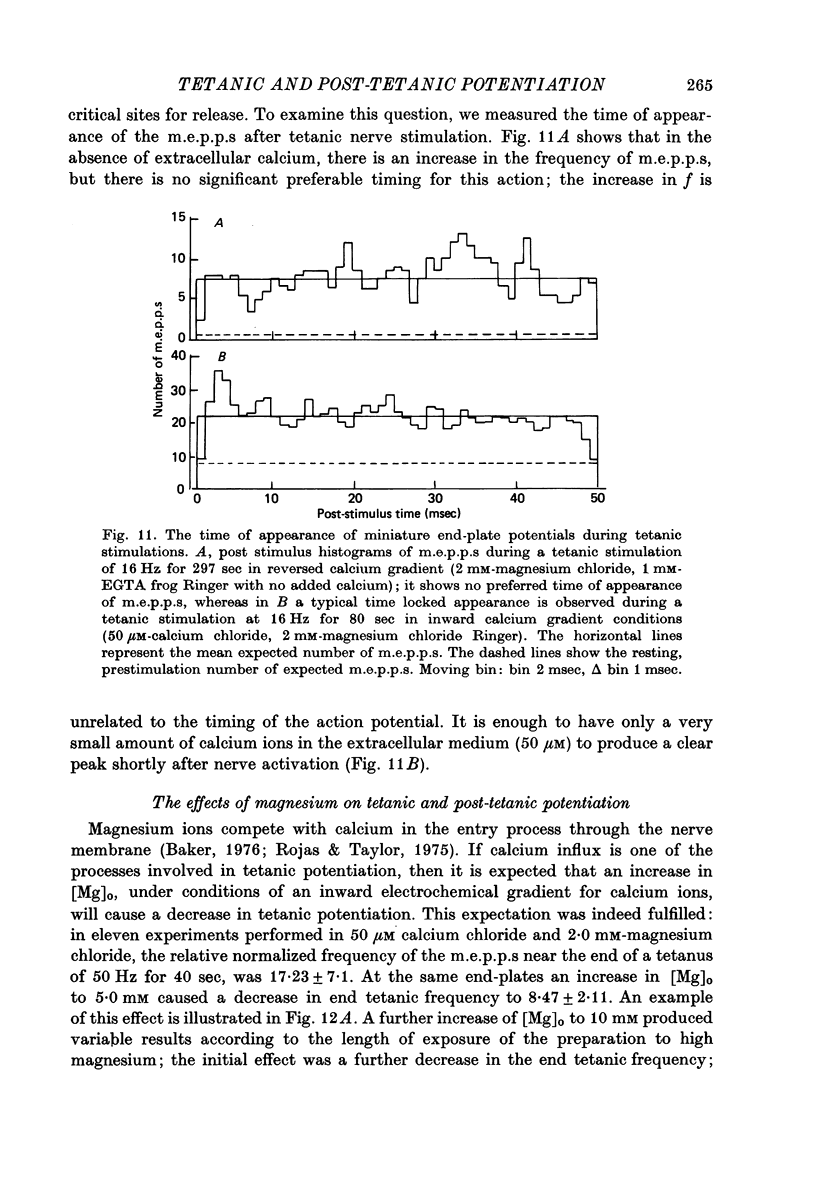
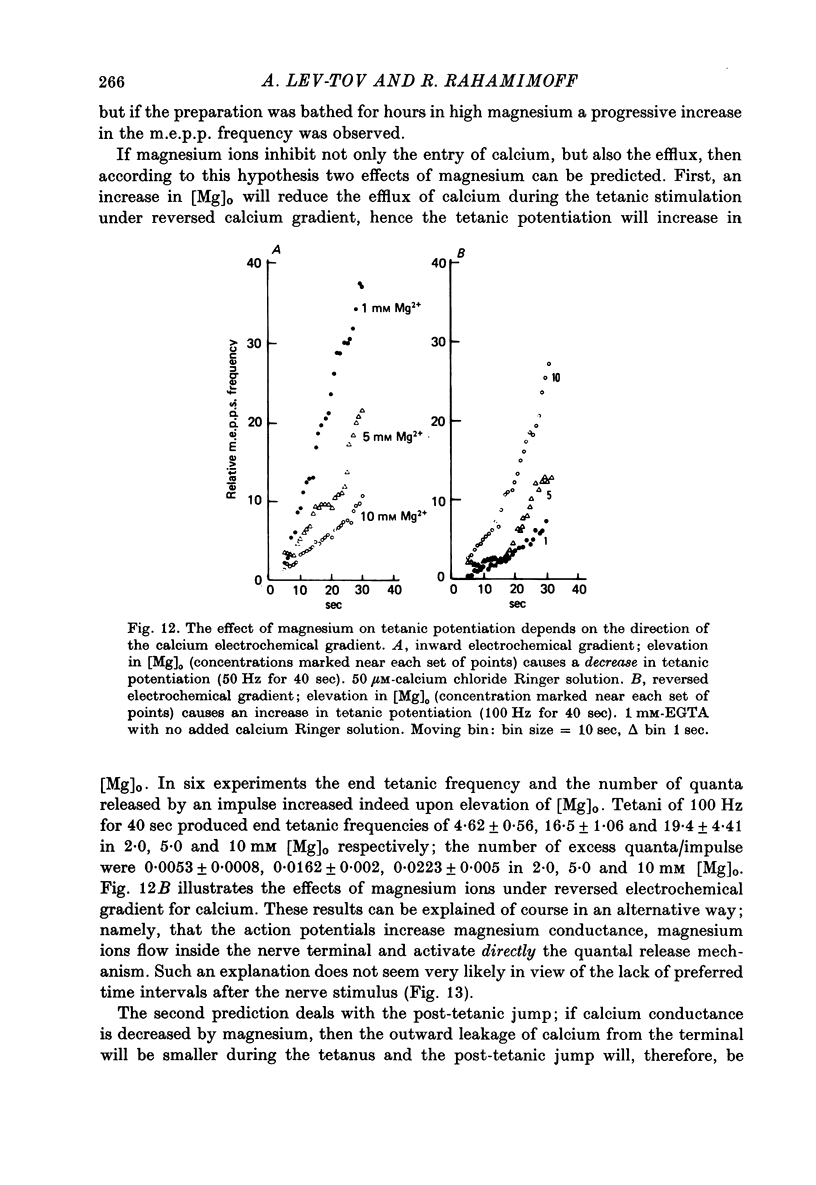
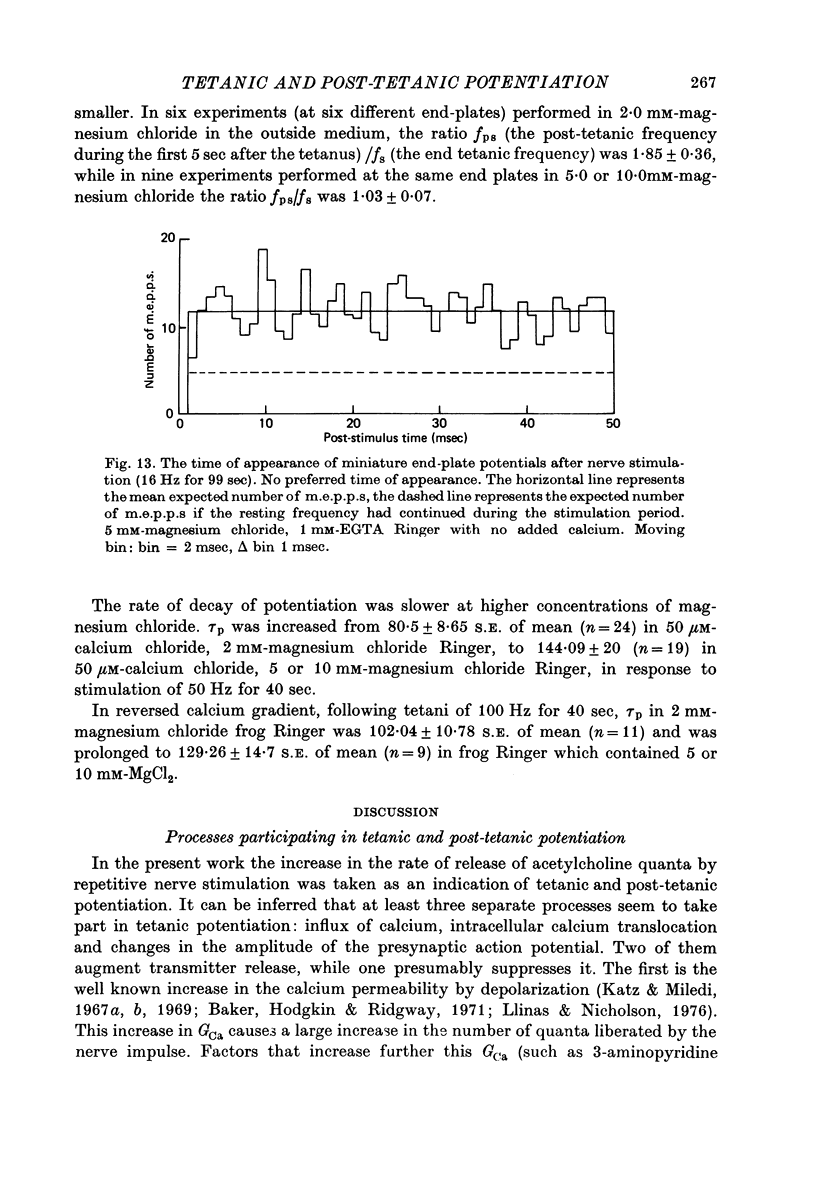
1. The involvement of calcium sodium, potassium and magnesium in tetanic and post-tetanic potentiation of miniature end-plate potential frequency was examined at the frog neuromuscular junction using conventional electrophysiological techniques. 2. Tetanic potentiation is larger in calcium containing solutions, than in solutions which generate reversed electrochemical gradient for calcium during nerve activity. 3. Tetanic potentiation increases with stimulation frequency and duration, under both inward and reversed electrochemical gradient for calcium conditions. This indicates that factors, other than calcium entry, participate in tetanic potentiation. 4. Addition of the potassium conductance blocking agent, 3-aminopyridine (5 mM), increases tetanic potentiation in calcium containing media, while depressing it under reversed calcium gradient. 5. Electronic depolarization of the nerve terminal in tetrodotoxin-containing Ringer solution, produces tetanic potentiation under inward gradient, but fails to do so under reversed gradient. This indicates that the entry of sodium ions participates in the generation of tetanic potentiation. 6. Addition of magnesium ions suppresses tetanic potentiation in calcium containing solution, but increases tetanic potentiation under reversed gradient. 7. The results are explained by the hypothesis that calcium entry and intracellular calcium translocation participate in the generation of tetanic potentiation. 8. Both the fast and the slow components (augmentation and potentiation respectively) of post-tetanic potentiation increase in duration, with increase in the tetanic stimulation rate. 9. The decay of post-tetanic potentiation increases: when [Ca]o is elevated by ionophoretic application during the decay phase only, when ouabain is present in the medium or when [Mg]o is elevated. These finding suggest that calcium, sodium and possibly magnesium take part in post-tetanic potentiation.
Full text
PDF


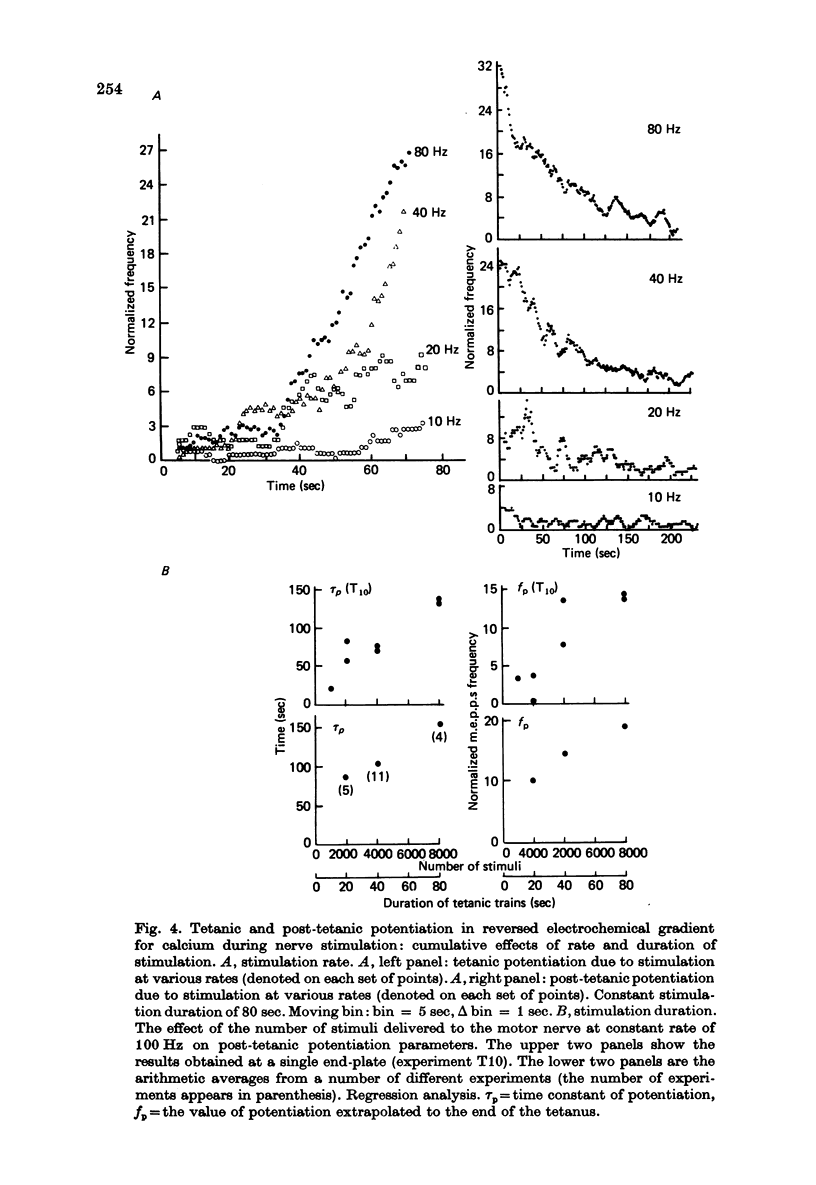

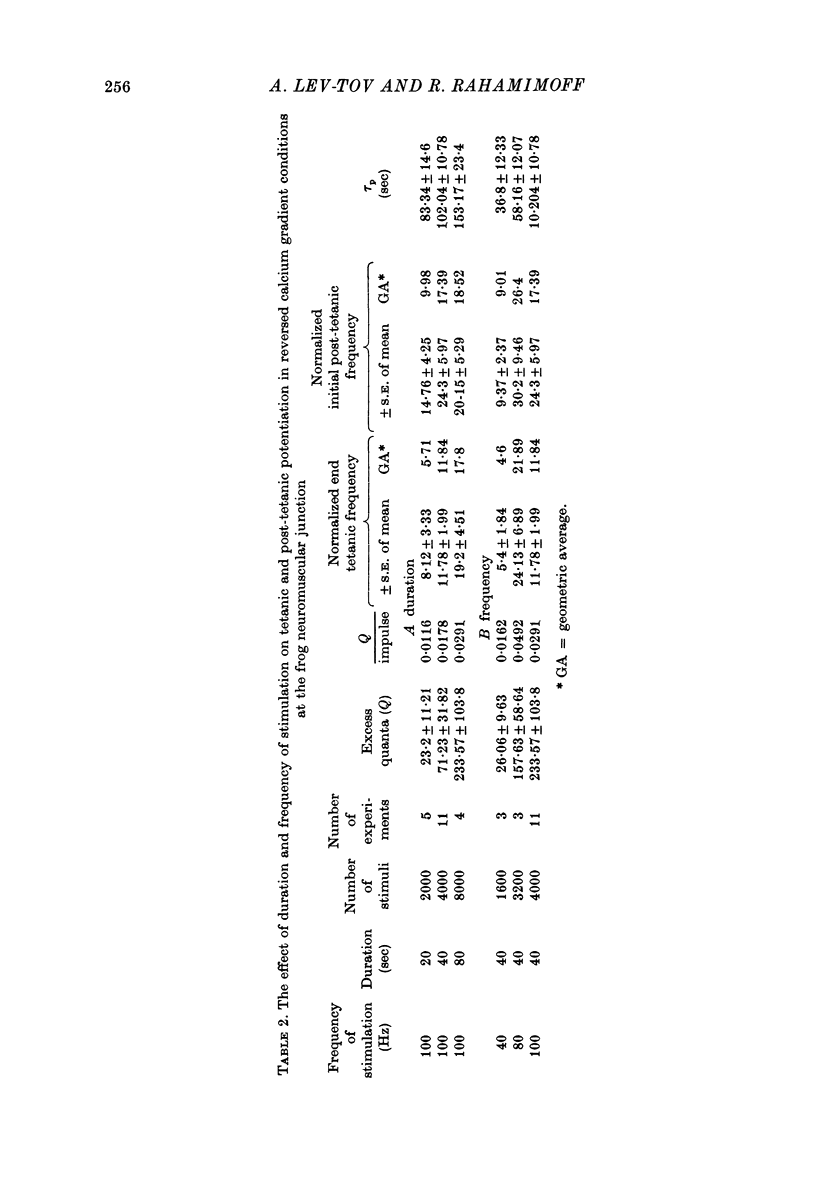
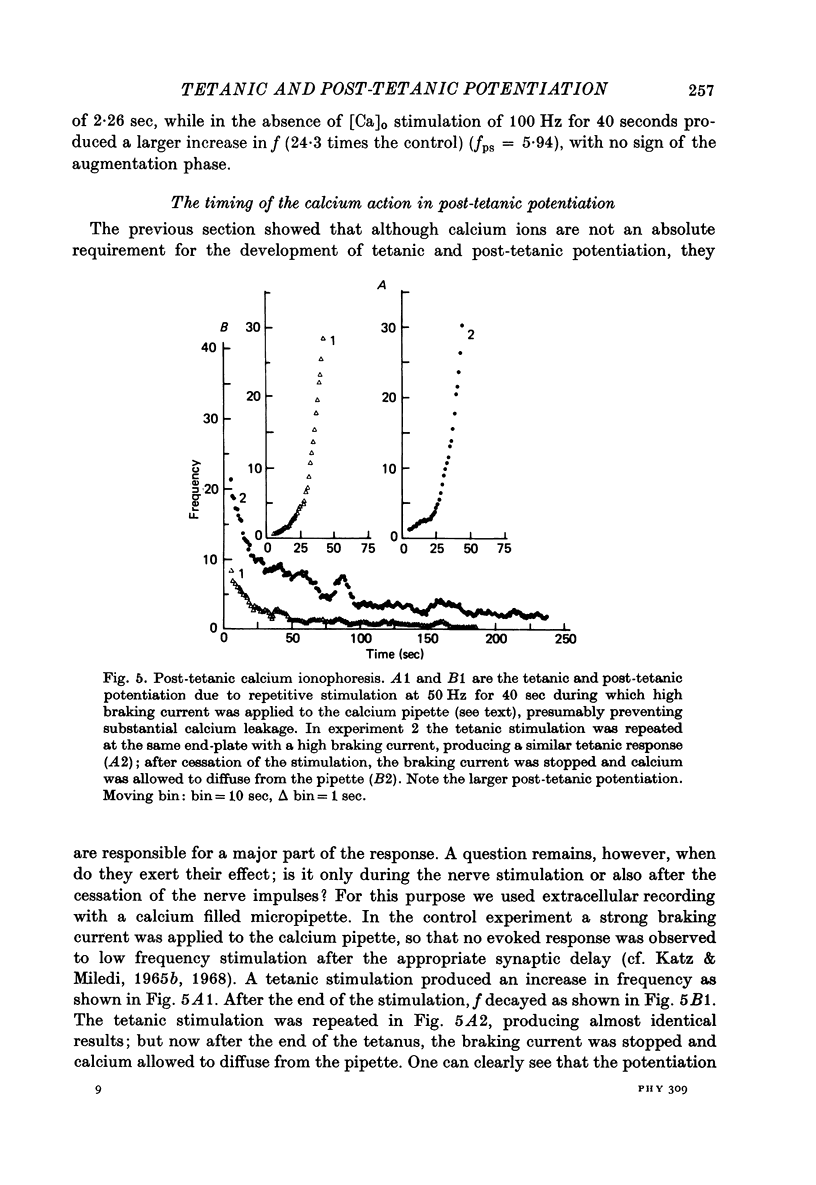

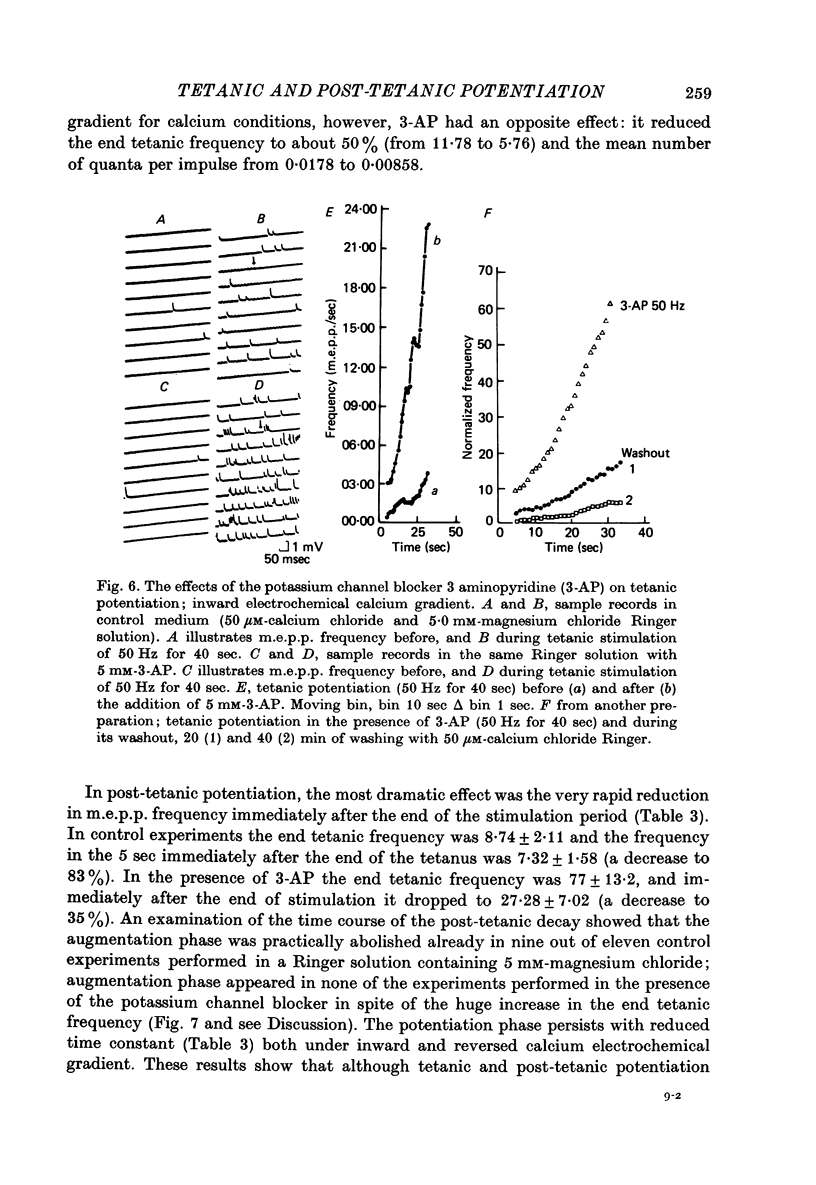







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnaes E., Rahamimoff R. On the role of mitochondria in transmitter release from motor nerve terminals. J Physiol. 1975 Jun;248(2):285–306. doi: 10.1113/jphysiol.1975.sp010974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood H. L., Swenarchuk L. E., Gruenwald C. R. Long-term synaptic facilitation during sodium accumulation in nerve terminals. Brain Res. 1975 Dec 12;100(1):198–202. doi: 10.1016/0006-8993(75)90260-7. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. A note of the mechanism by which inhibitors of the sodium pump accelerate spontaneous release of transmitter from motor nerve terminals. J Physiol. 1975 May;247(1):209–226. doi: 10.1113/jphysiol.1975.sp010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. Sodium-dependent transport of magnesium ions in giant axons of Loligo forbesi. J Physiol. 1971 Jul;216(1):38P–40P. [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. The influence of extracellular calcium binding on the calcium efflux from squid axons. J Physiol. 1978 Mar;276:127–150. doi: 10.1113/jphysiol.1978.sp012223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Calcium entry in response to maintained depolarization of squid axons. J Physiol. 1973 Jun;231(3):527–548. doi: 10.1113/jphysiol.1973.sp010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Regulation of intracellular Ca and Mg in squid axons. Fed Proc. 1976 Dec;35(14):2589–2595. [PubMed] [Google Scholar]
- Baker P. F., Schlaepfer W. W. Uptake and binding of calcium by axoplasm isolated from giant axons of Loligo and Myxicola. J Physiol. 1978 Mar;276:103–125. doi: 10.1113/jphysiol.1978.sp012222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Martin A. R., Rahamimoff R. A note on the interaction of spontaneous and evoked release at the frog neuromuscular junction. J Physiol. 1974 Mar;237(2):453–463. doi: 10.1113/jphysiol.1974.sp010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The action of sodium pump inhibitors on neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):381–399. doi: 10.1098/rspb.1968.0046. [DOI] [PubMed] [Google Scholar]
- Birks R. I., Cohen M. W. The influence of internal sodium on the behaviour of motor nerve endings. Proc R Soc Lond B Biol Sci. 1968 Jul 9;170(1021):401–421. doi: 10.1098/rspb.1968.0047. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Ratzlaff R. W., Kendrick N. C., Schweitzer E. S. Calcium buffering in presynaptic nerve terminals. I. Evidence for involvement of a nonmitochondrial ATP-dependent sequestration mechanism. J Gen Physiol. 1978 Jul;72(1):15–41. doi: 10.1085/jgp.72.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M., Schmidt R. F. Potential changes recorded from the frog motor nerve terminal during its activation. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;287(1):56–80. doi: 10.1007/BF00362454. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Crompton M. The regulation of intracellular calcium by mitochondria. Ann N Y Acad Sci. 1978 Apr 28;307:269–284. doi: 10.1111/j.1749-6632.1978.tb41957.x. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Statistical factors involved in neuromuscular facilitation and depression. J Physiol. 1954 Jun 28;124(3):574–585. doi: 10.1113/jphysiol.1954.sp005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE F. A., FRANKENHAEUSER B. Membrane currents in isolated frog nerve fibre under voltage clamp conditions. J Physiol. 1958 Aug 29;143(1):76–90. doi: 10.1113/jphysiol.1958.sp006045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Physiological role of ATP-driven calcium pump in squid axon. Nature. 1979 Mar 15;278(5701):271–273. doi: 10.1038/278271a0. [DOI] [PubMed] [Google Scholar]
- DiPolo R. Ca pump driven by ATP in squid axons. Nature. 1978 Jul 27;274(5669):390–392. doi: 10.1038/274390a0. [DOI] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Miledi R., Rahamimoff R. Strontium and quantal release of transmitter at the neuromuscular junction. J Physiol. 1969 Jan;200(1):267–283. doi: 10.1113/jphysiol.1969.sp008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Rahamimoff R., Rotshenker S. Quelling of spontaneous transmitter release by nerve impulses in low extracellular calcium solutions. J Physiol. 1978 May;278:491–500. doi: 10.1113/jphysiol.1978.sp012319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Rahamimoff R. The role of calcium ions in tetanic and post-tetanic increase of miniature end-plate potential frequency. J Physiol. 1978 May;278:501–511. doi: 10.1113/jphysiol.1978.sp012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. REPETITIVE STIMULATION AT THE MAMMALIAN NEUROMUSCULAR JUNCTION, AND THE MOBILIZATION OF TRANSMITTER. J Physiol. 1963 Dec;169:641–662. doi: 10.1113/jphysiol.1963.sp007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ozawa S., Sand O. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol. 1975 May;65(5):617–644. doi: 10.1085/jgp.65.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart M. P., Reese T. S., Brinley F. J., Jr Endoplasmic reticulum sequesters calcium in the squid giant axon. Science. 1978 Dec 22;202(4374):1300–1303. doi: 10.1126/science.725607. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut W. P., Longenecker H. B., Jr, Mauro A. Effects of calcium and magnesium on the frequency of miniature end-plate potentials during prolonged tetanization. J Physiol. 1971 Dec;219(1):17–38. doi: 10.1113/jphysiol.1971.sp009647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON D. H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J Physiol. 1957 Oct 30;138(3):434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959 Dec;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenskaya M. A., Elmqvist D., Thesleff S. Guanidine and neuromuscular transmission. II. Effect on transmitter release in response to repetitive nerve stimulation. Arch Neurol. 1975 Aug;32(8):510–518. doi: 10.1001/archneur.1975.00490500030002. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):8–22. doi: 10.1098/rspb.1967.0010. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The release of acetylcholine from nerve endings by graded electric pulses. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):23–38. doi: 10.1098/rspb.1967.0011. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2918–2922. doi: 10.1073/pnas.73.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. A quantitative description of tetanic and post-tetanic potentiation of transmitter release at the frog neuromuscular junction. J Physiol. 1975 Feb;245(1):183–208. doi: 10.1113/jphysiol.1975.sp010840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. Augmentation: A process that acts to increase transmitter release at the frog neuromuscular junction. J Physiol. 1976 May;257(2):449–470. doi: 10.1113/jphysiol.1976.sp011378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 1973 Aug;233(1):127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgo J., Lemeignan M., Lechat P. Modifications de la libération du transmetteur à la jonction neuromusculaire de grenouille sous l'action de l'amino-4 pyridine. C R Acad Sci Hebd Seances Acad Sci D. 1975 Nov 24;281(21):1637–1639. [PubMed] [Google Scholar]
- Politoff A. L., Rose S., Pappas G. D. The calcium binding sites of synaptic vesicles of the frog sartorius neuromuscular junction. J Cell Biol. 1974 Jun;61(3):818–823. doi: 10.1083/jcb.61.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff H., Abramovitz E. Ca transport and ATPase activity of synaptosomal vesicles from rat brain. FEBS Lett. 1978 Aug 15;92(2):163–167. doi: 10.1016/0014-5793(78)80745-5. [DOI] [PubMed] [Google Scholar]
- Rahamimoff H., Abramovitz E. Calcium transport in a vesicular membrane preparation from rat brain synaptosomes. FEBS Lett. 1978 May 15;89(2):223–226. doi: 10.1016/0014-5793(78)80222-1. [DOI] [PubMed] [Google Scholar]
- Rojas E., Taylor R. E. Simultaneous measurements of magnesium, calcium and sodium influxes in perfused squid giant axons under membrane potential control. J Physiol. 1975 Oct;252(1):1–27. doi: 10.1113/jphysiol.1975.sp011131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J. Post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1969 Jul;203(1):121–133. doi: 10.1113/jphysiol.1969.sp008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotshenker S., Erulkar S. D., Rahamimoff R. Reduction in the frequency of miniature end-plate potentials by nerve stimulation in low calcium solutions. Brain Res. 1976 Jan 16;101(2):362–365. doi: 10.1016/0006-8993(76)90277-8. [DOI] [PubMed] [Google Scholar]
- Swenarchuk L. E., Atwood H. L. Long-term synaptic facilitation with minimal calcium entry. Brain Res. 1975 Dec 12;100(1):205–208. doi: 10.1016/0006-8993(75)90261-9. [DOI] [PubMed] [Google Scholar]
- Ulbricht W., Wagner H. H. Block of potassium channels of the nodal membrane by 4-aminopyridine and its partial removal on depolarization. Pflugers Arch. 1976 Nov 30;367(1):77–87. doi: 10.1007/BF00583659. [DOI] [PubMed] [Google Scholar]
- Weinreich D. Ionic mechanism of post-tetanic potentiation at the neuromuscular junction of the frog. J Physiol. 1971 Jan;212(2):431–446. doi: 10.1113/jphysiol.1971.sp009333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Dynamics of aminopyridine block of potassium channels in squid axon membrane. J Gen Physiol. 1976 Nov;68(5):519–535. doi: 10.1085/jgp.68.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


