Abstract
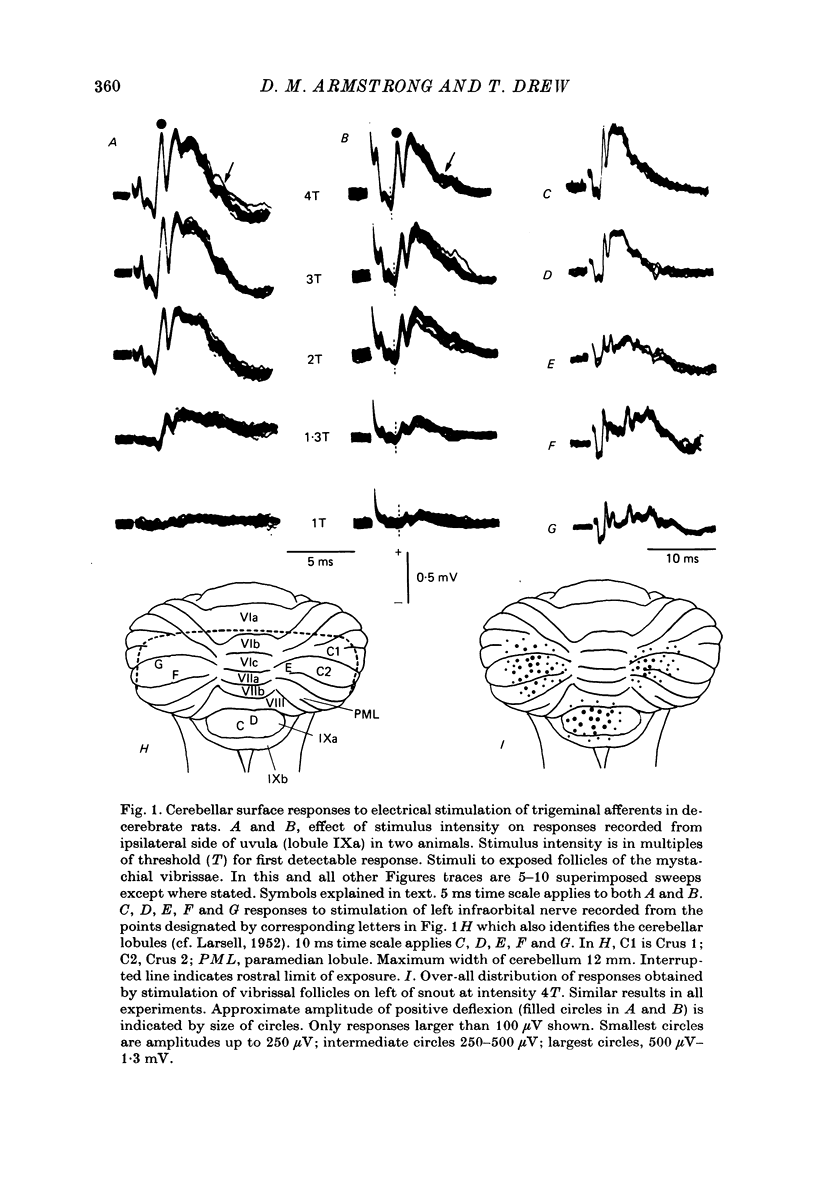
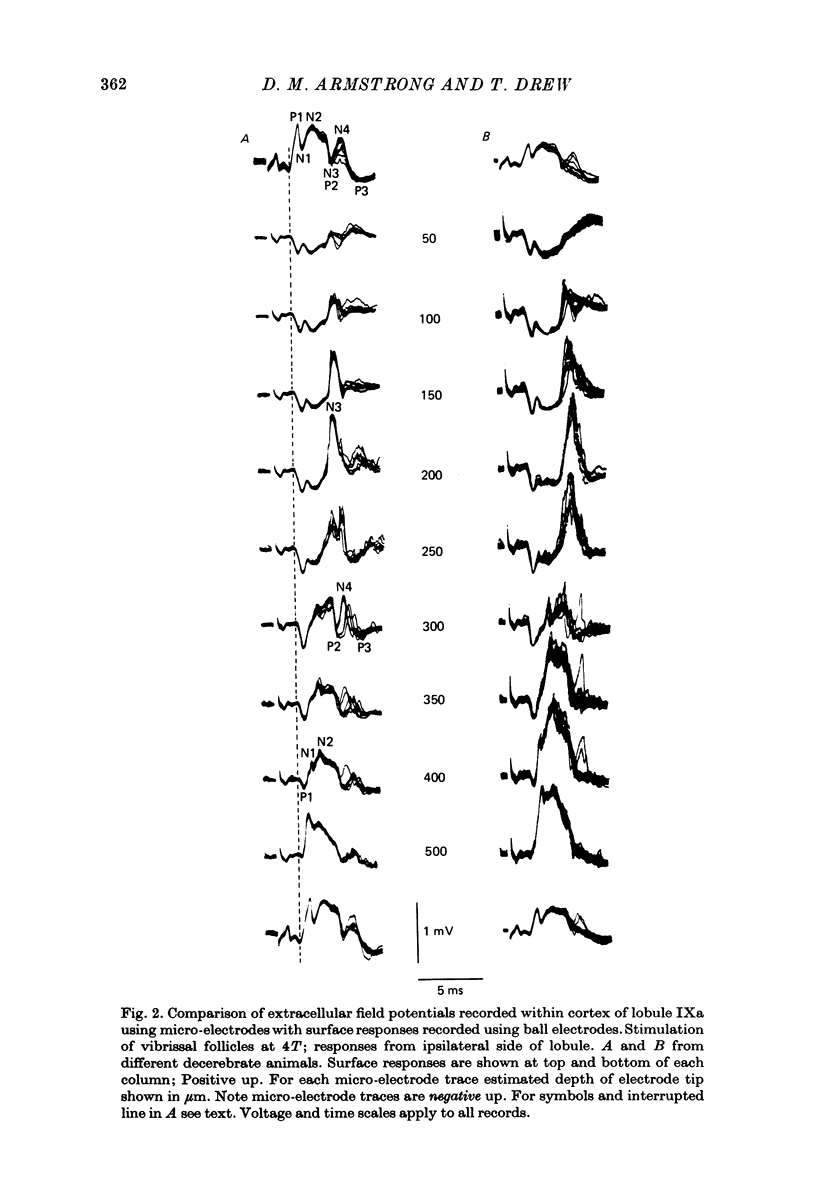
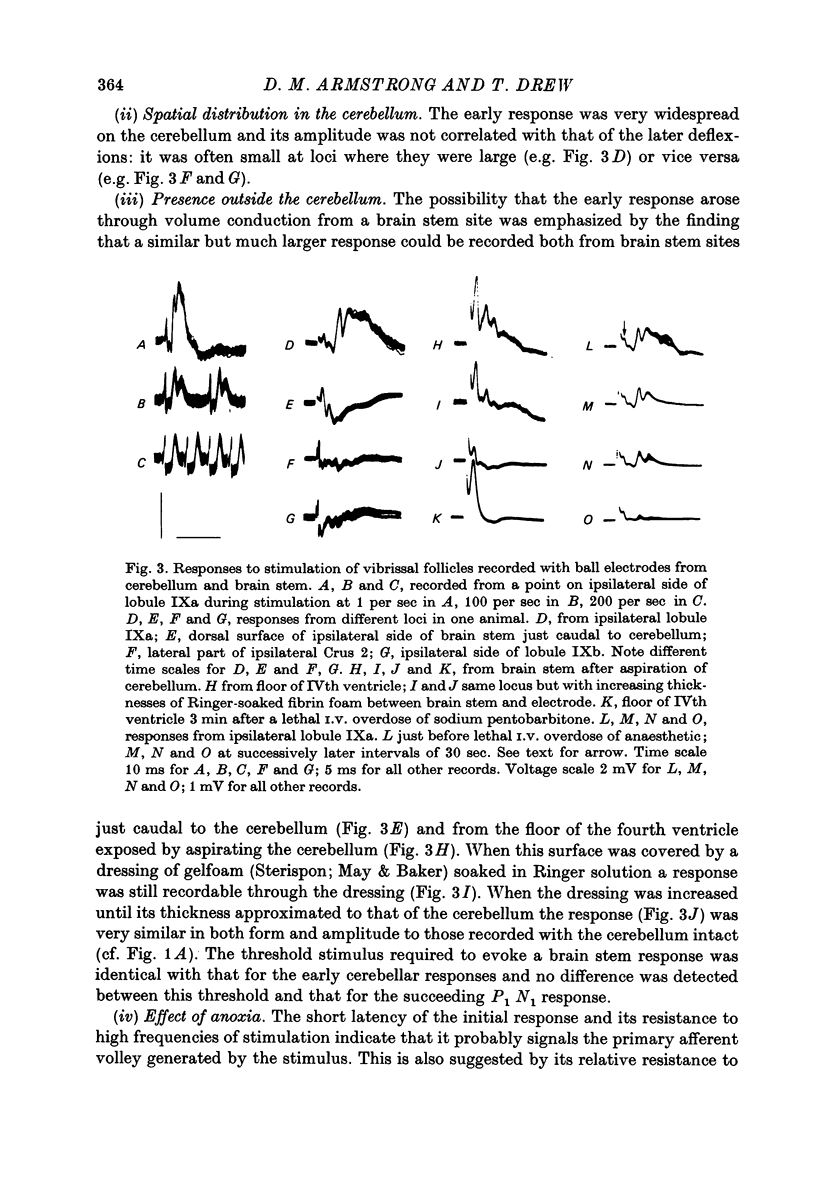
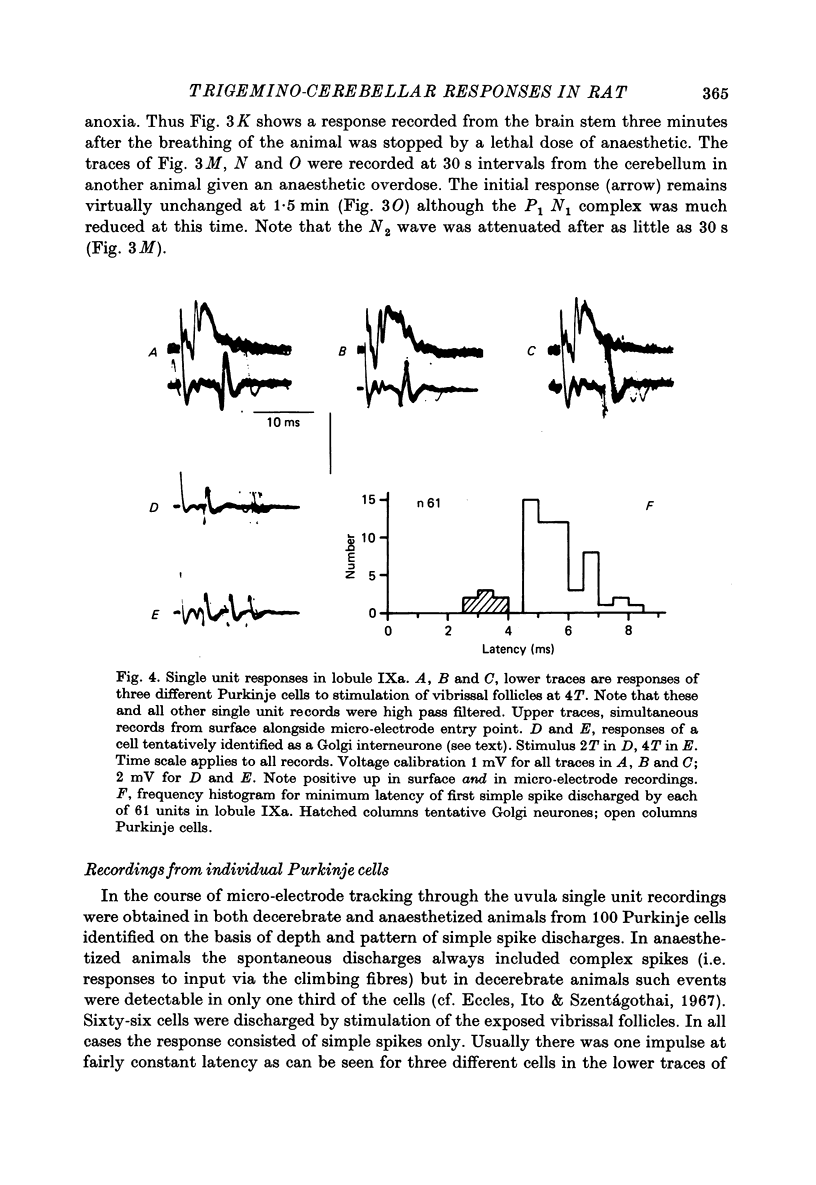
1. Responses in the cortex of the posterior lobe of the cerebellum to electrical stimulation of afferent fibres from the skin of the snout have been analysed in decerebrate and pentobarbitone-anaesthetized rats by means of surface and microelectrode records. Single shocks were applied either to the exposed follicles of the mystachial vibrissae or to the infraorbital branch of the trigeminal nerve. 2. In decerebrate rats responses were mediated only via mossy fibre afferents. Stimulation of one side of the snout yielded responses with mean latency 2.4 ms throughout the uvula (largest ipsilaterally and in lobule IXa). Smaller responses with similar latency were present in both cerebellar hemispheres (largest ipsilaterally). The earliest discharges of Purkinje cells in lobule IXa occurred at latencies between 4.5 and 8.5 ms. 3. All components of the extracellular field potentials generated within the cortex by the mossy fibre input were detectable by surface recording with ball electrodes. 4. The earliest surface potentials had a latency of 0.55 ms (peak latency 0.8 ms); they arose through volume conduction from the brain stem of a potential which signalled arrival of the primary afferent volley. The short delay between this event and the arrival of the mossy fibre volley in the cerebellum suggests that only one synaptic relay occurs in the brain stem. 5. In pentobarbitone-anaesthetized rats surface responses mediated via mossy fibres persisted and were accompanied at slightly higher threshold by responses shown to be mediated via climbing fibres. The latter were present in descending order of amplitude in three sagittally directed zones, one in contralateral Crus 2 (minimum latency 13 ms), one in the vermis contralaterally near the mid line in lobule IXa (latency 16 ms) and a third in ipsilateral Crus 2 (latency 20 ms) 6. In the hemisphere the responses mediated via climbing fibres occurred within the somewhat larger zones activated via mossy fibres but in the vermis the two types of trigemino-cerebellar input influenced quite separate areas of cortex.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M. Functional significance of connections of the inferior olive. Physiol Rev. 1974 Apr;54(2):358–417. doi: 10.1152/physrev.1974.54.2.358. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Schild R. F. An investigation of the cerebellar cortico-nuclear projections in the rat using an autoradiographic tracing method. I. Projections from the vermis. Brain Res. 1978 Feb 3;141(1):1–19. doi: 10.1016/0006-8993(78)90613-3. [DOI] [PubMed] [Google Scholar]
- Axelrad H., Crepel F. Représentation sélective des vibrisses mystaciales au niveau des cellules de Purkinje du cervelet par la voie des fibres grimpantes chez le rat. C R Acad Sci Hebd Seances Acad Sci D. 1977 Apr 4;284(14):1321–1324. [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L., Brown J. T., Van Itallie C. Sagittal organization of olivocerebellar and reticulocerebellar projections: autoradiographic studies with 35S-methionine. Exp Brain Res. 1977 Dec 19;30(4):561–576. doi: 10.1007/BF00237645. [DOI] [PubMed] [Google Scholar]
- Cody F. W., Richardson H. C. Mossy and climbing fibre mediated responses evoked in the cerebellar cortex of the cat by trigeminal afferent stimulation. J Physiol. 1979 Feb;287:1–14. doi: 10.1113/jphysiol.1979.sp012641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. R., Wiesendanger M. Input from trigeminal cutaneous afferents to neurones of the inferior olive in rats. Exp Brain Res. 1976 Sep 24;26(2):193–202. doi: 10.1007/BF00238283. [DOI] [PubMed] [Google Scholar]
- DARIAN-SMITH I., PHILLIPS G. SECONDARY NEURONES WITHIN A TRIGEMINO-CEREBELLAR PROJECTION TO THE ANTERIOR LOBE OF THE CEREBELLUM IN THE CAT. J Physiol. 1964 Jan;170:53–68. doi: 10.1113/jphysiol.1964.sp007313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. doi: 10.1007/BF00235207. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The inhibitory interneurones within the cerebellar cortex. Exp Brain Res. 1966;1(1):1–16. doi: 10.1007/BF00235206. [DOI] [PubMed] [Google Scholar]
- Gordon M., Rubia F. J., Strata P. The effect of pentothal on the activity evoked in the cerebellar cortex. Exp Brain Res. 1973 Mar 29;17(1):50–62. doi: 10.1007/BF00234563. [DOI] [PubMed] [Google Scholar]
- Groenewegen H. J., Voogd J., Freedman S. L. The parasagittal zonation within the olivocerebellar projection. II. Climbing fiber distribution in the intermediate and hemispheric parts of cat cerebellum. J Comp Neurol. 1979 Feb 1;183(3):551–601. doi: 10.1002/cne.901830307. [DOI] [PubMed] [Google Scholar]
- Groenewegen H. J., Voogd J. The parasagittal zonation within the olivocerebellar projection. I. Climbing fiber distribution in the vermis of cat cerebellum. J Comp Neurol. 1977 Aug 1;174(3):417–488. doi: 10.1002/cne.901740304. [DOI] [PubMed] [Google Scholar]
- Joseph J. W., Shambes G. M., Gibson J. M., Welker W. Tactile projections to granule cells in caudal vermis of the rat's cerebellum. Brain Behav Evol. 1978;15(2):141–149. doi: 10.1159/000123776. [DOI] [PubMed] [Google Scholar]
- LARSELL O. The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J Comp Neurol. 1952 Oct;97(2):281–356. doi: 10.1002/cne.900970204. [DOI] [PubMed] [Google Scholar]
- Latham A., Paul D. H. Effects of sodium thiopentone on cerebellar neurone activity. Brain Res. 1971 Jan 8;25(1):212–215. doi: 10.1016/0006-8993(71)90585-3. [DOI] [PubMed] [Google Scholar]
- Miles T. S., Wiesendanger M. Climbing fibre inputs to cerebellar Purkinje cells from trigeminal cutaneous afferents and the SI face area of the cerebral cortex in the cat. J Physiol. 1975 Feb;245(2):425–445. doi: 10.1113/jphysiol.1975.sp010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles T. S., Wiesendanger M. Organization of climbing fibre projections to the cerebellar cortex from trigeminal cutaneous afferents and from the SI face area of the cerebral cortex in the cat. J Physiol. 1975 Feb;245(2):409–424. doi: 10.1113/jphysiol.1975.sp010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn A. C., Zuber B. L. Cerebellar evoked potentials resulting from extraocular muscle stretch: evidence against a cerebellar origin. Exp Neurol. 1971 May;31(2):230–238. doi: 10.1016/0014-4886(71)90192-0. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Strata P. Responses evoked in the cerebellar cortex by stimulating mossy fibre pathways to the cerebellum. Exp Brain Res. 1967;3(2):95–110. doi: 10.1007/BF00233255. [DOI] [PubMed] [Google Scholar]
- Shambes G. M., Beermann D. H., Welker W. Multiple tactile areas in cerebellar cortex: another patchy cutaneous projection to granule cell columns in rats. Brain Res. 1978 Nov 17;157(1):123–128. doi: 10.1016/0006-8993(78)91000-4. [DOI] [PubMed] [Google Scholar]
- Shambes G. M., Gibson J. M., Welker W. Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol. 1978;15(2):94–140. doi: 10.1159/000123774. [DOI] [PubMed] [Google Scholar]
- Waite P. M. Somatotopic organization of vibrissal responses in the ventro-basal complex of the rat thalamus. J Physiol. 1973 Jan;228(2):527–540. doi: 10.1113/jphysiol.1973.sp010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971 Mar 5;26(2):259–275. [PubMed] [Google Scholar]
- Welker C. Receptive fields of barrels in the somatosensory neocortex of the rat. J Comp Neurol. 1976 Mar 15;166(2):173–189. doi: 10.1002/cne.901660205. [DOI] [PubMed] [Google Scholar]


