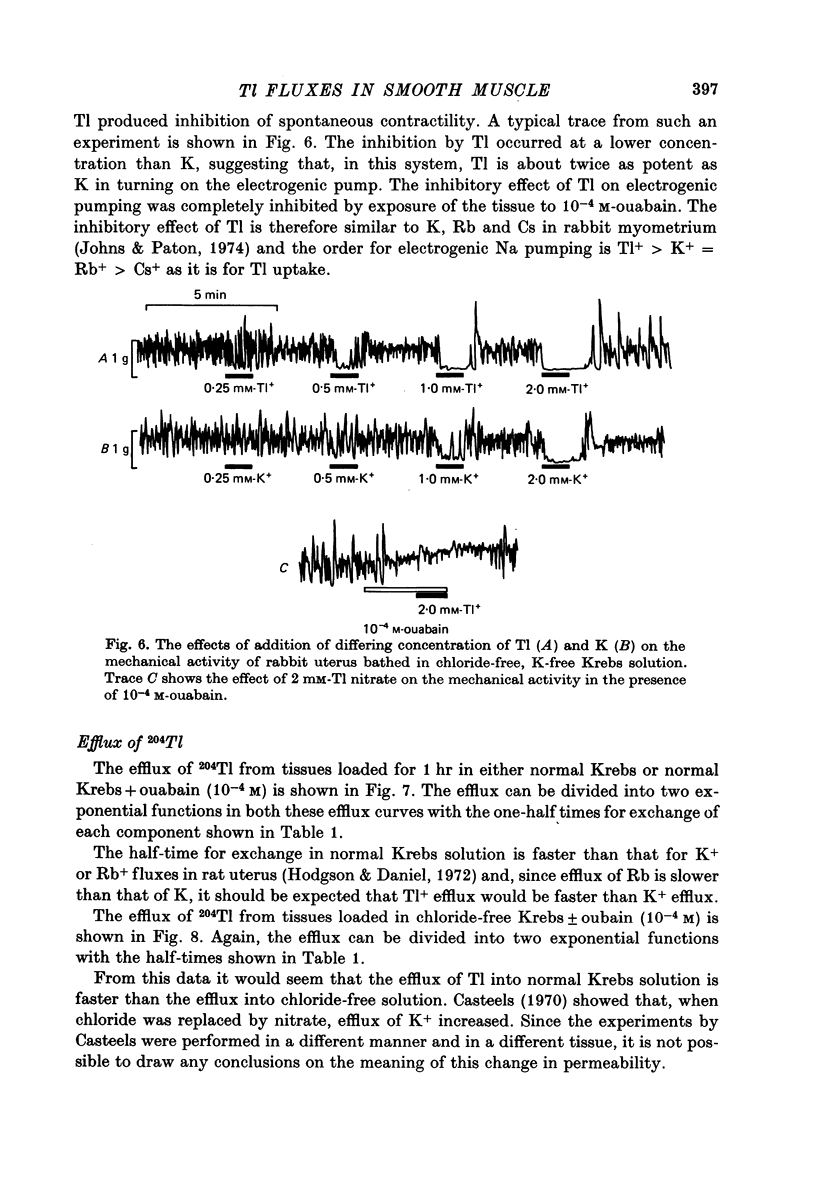
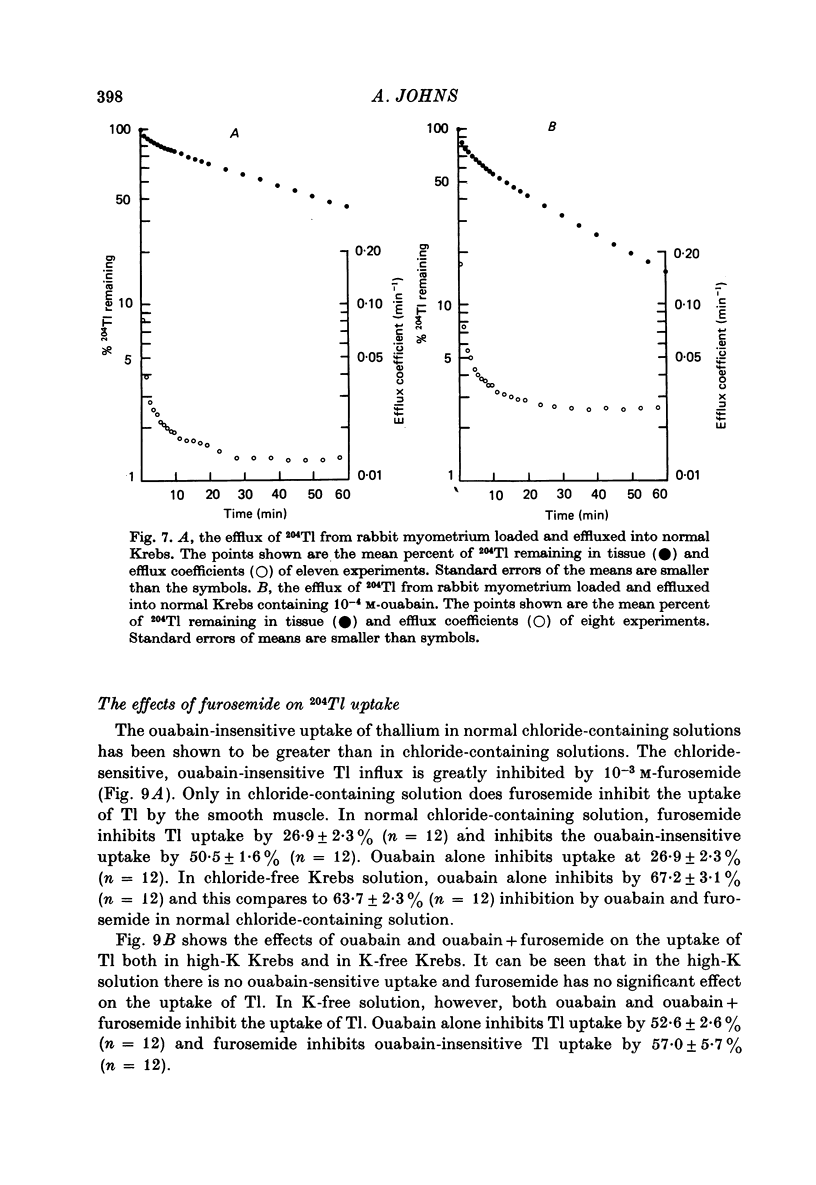
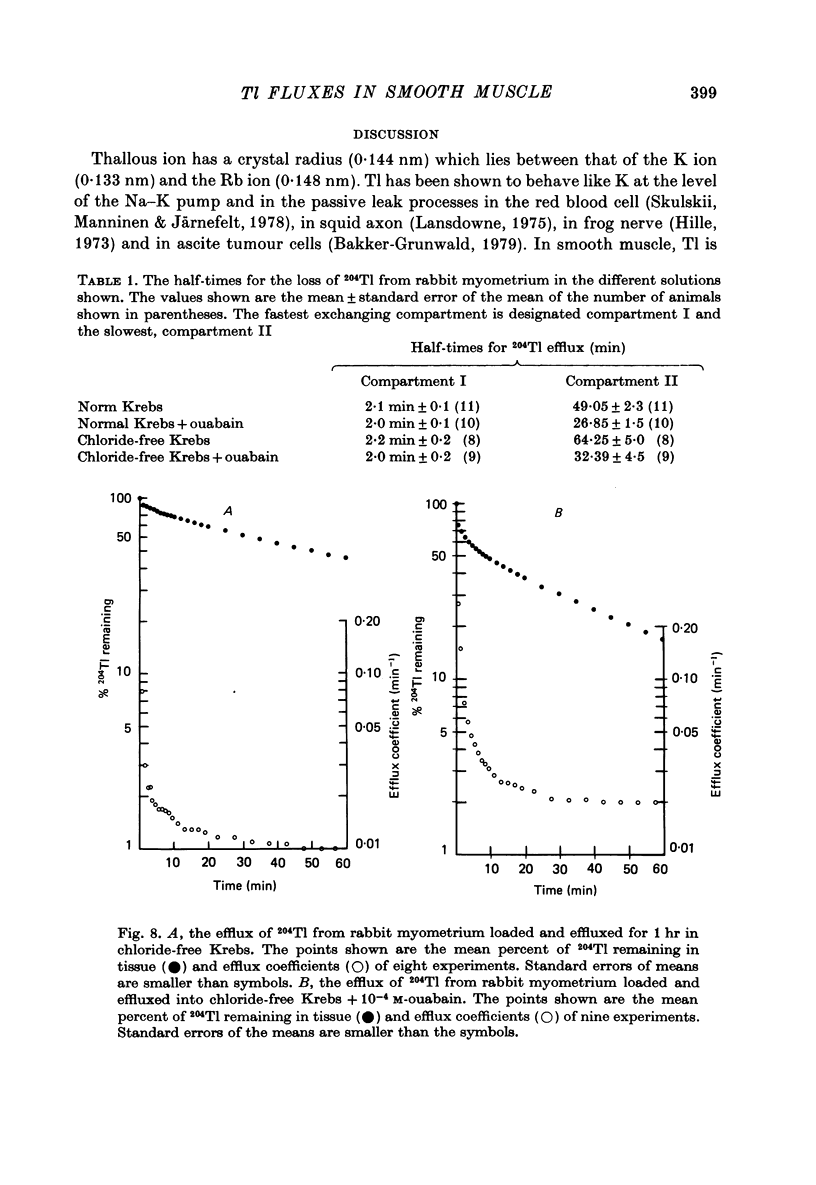
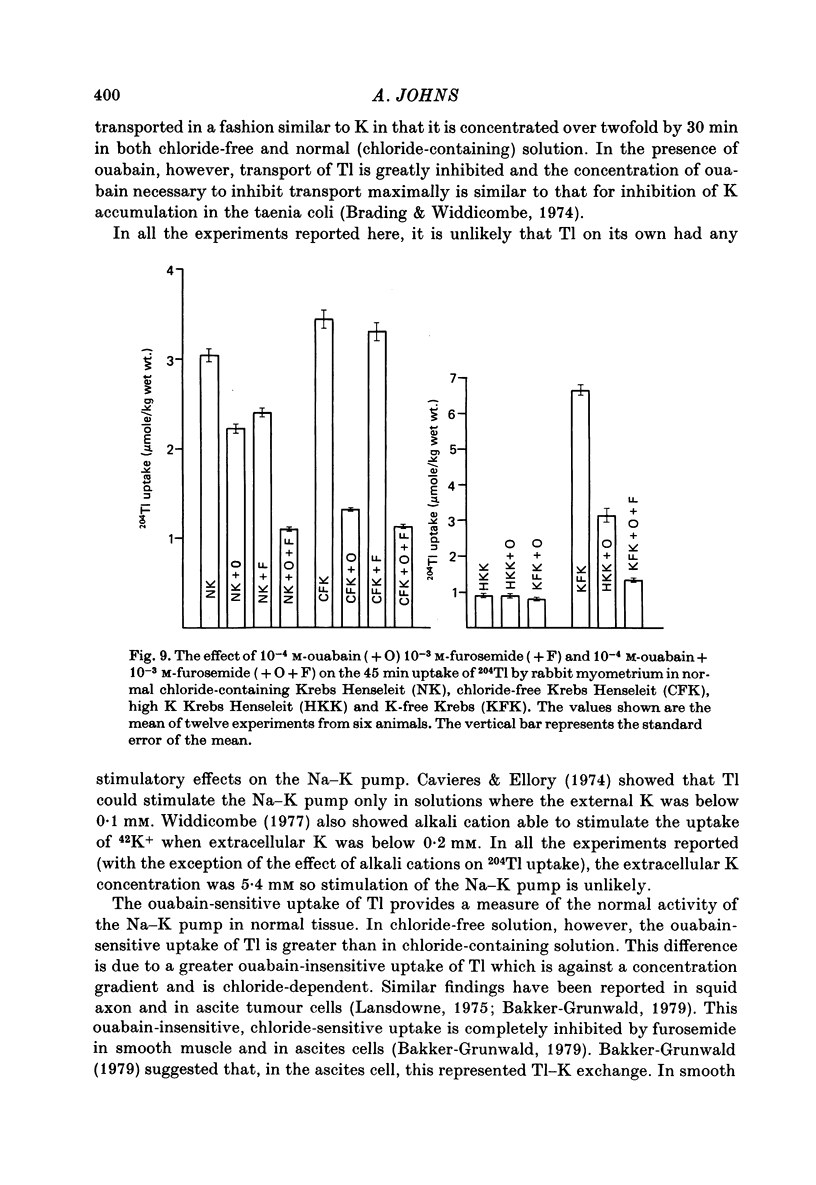
Abstract
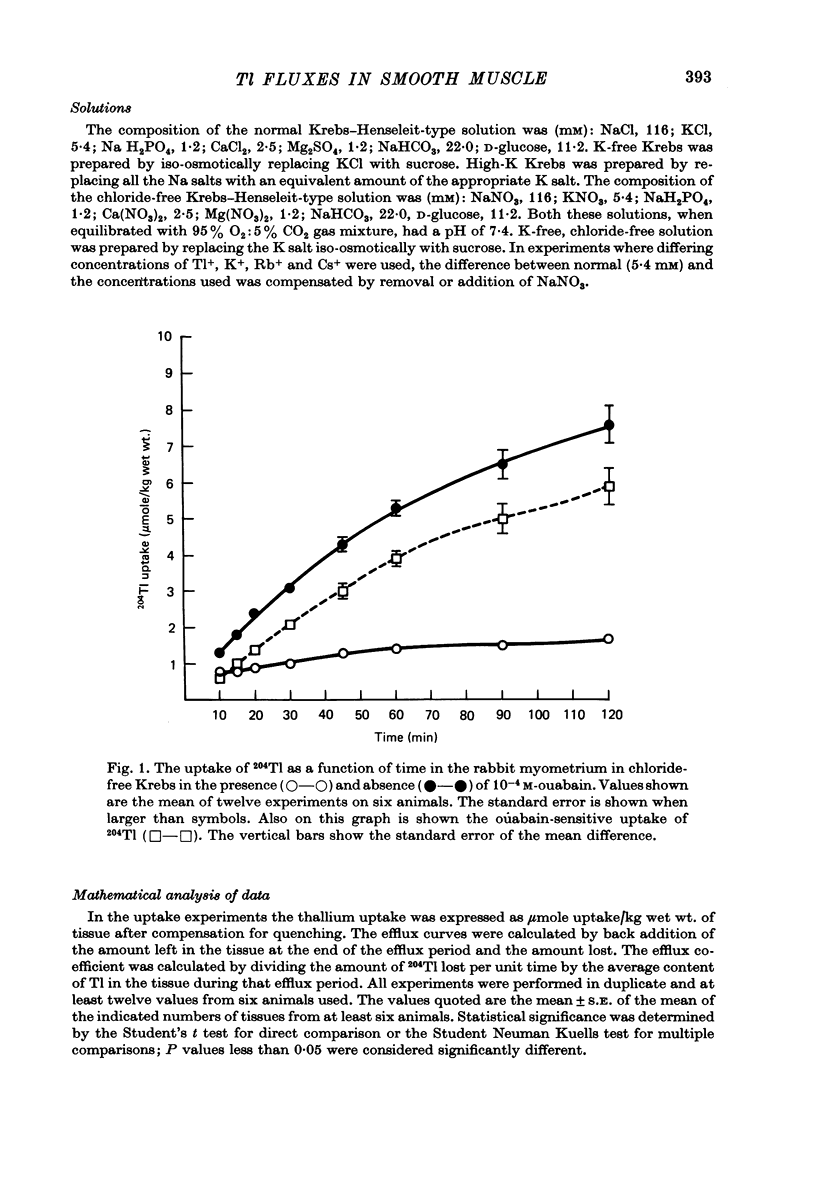
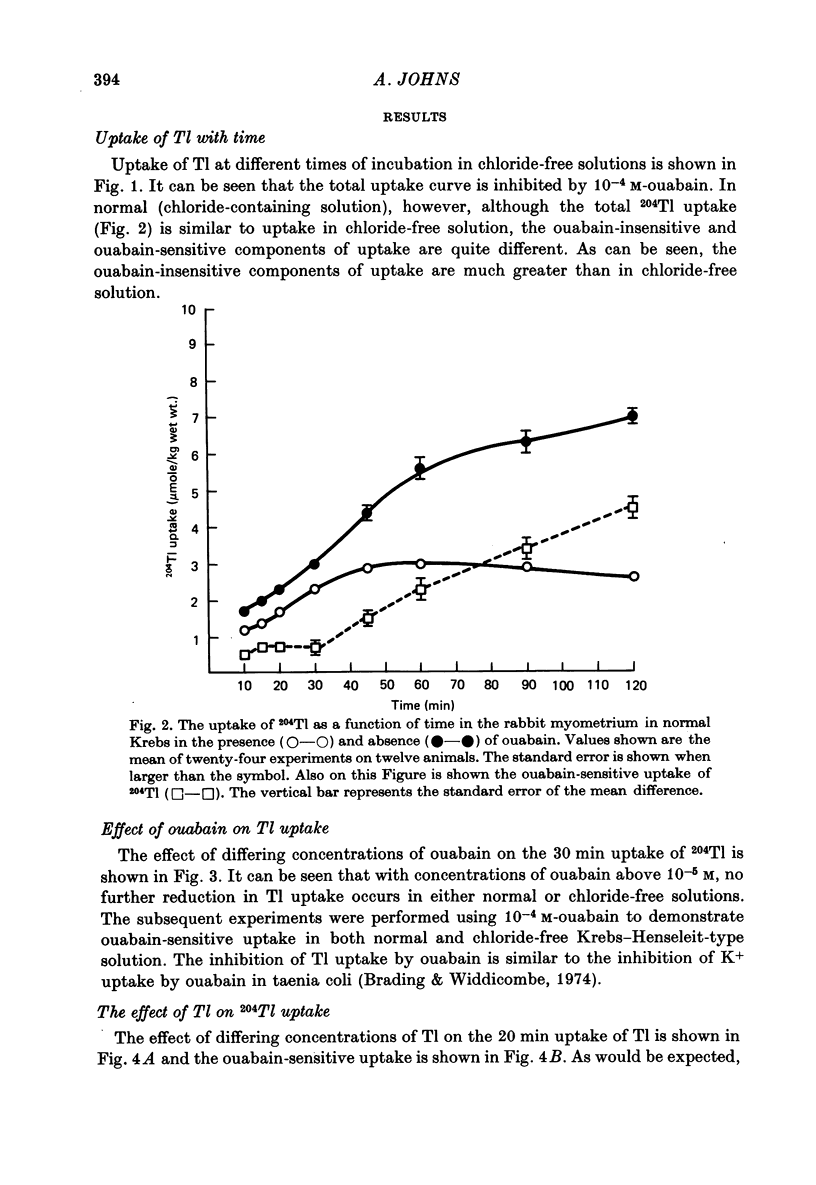
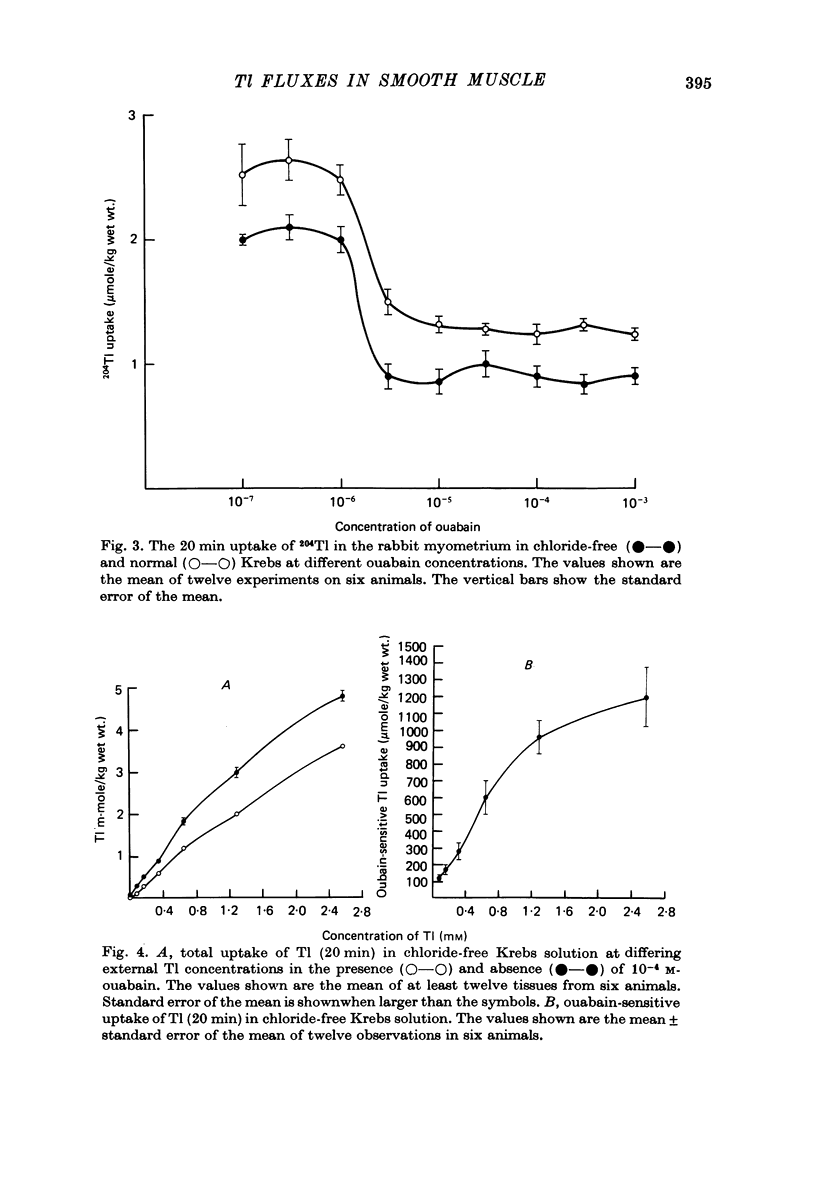
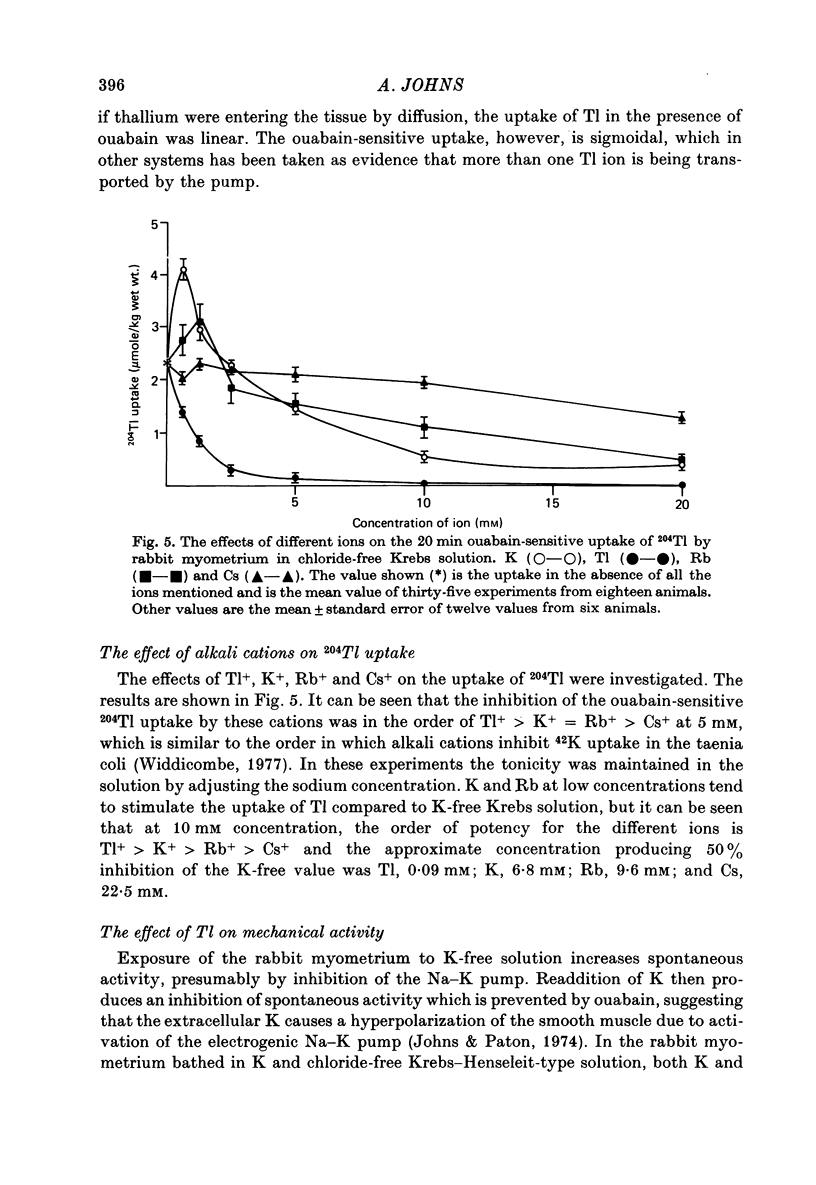
1. Rabbit myometrium accumulates Tl in a time-dependent fashion and the majority of the uptake of Tl is ouabain-sensitive. 2. In normal chloride-containing media, the uptake of Tl, though ouabain-sensitive, is less than in chloride-free media and this difference is due to a greater ouabain-insensitive uptake. 3. The ouabain-insensitive uptake in normal chloride-containing media is reduced by furosemide and furosemide also reduces total uptake in this solution. In chloride-free media, however, furosemide is without effect on total or ouabain-sensitive uptake. 4. In chloride-free media, the uptake of Tl against Tl concentration is sigmoidal, suggesting that more than one Tl ion is being transported at a time. 5. Tl was capable of substituting for K in electrogenic Na pumping; it was approximately twice as effective as K and the inhibitory effect of Tl was blocked by ouabain. 6. Tl efflux can be explained by a simple two-compartment model in both normal and chloride-free solutions. 7. The uptake of Tl was inhibited by alkali cations with the order of potency being Tl+ > K+ > Rb+ > Cs+ at 10 mM and Tl+ > K4 = Rb+ > Cs+ at 5 mM ion concentrations. 8. It is concluded that Tl enters the smooth muscle of rabbit uterus by diffusion, active ouabain-sensitive transport and active chloride- and furosemide-sensitive transport.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker-Grunwald T. Movement of thallous ion across the ascites cell membrane. J Membr Biol. 1979 May 21;47(2):171–183. doi: 10.1007/BF01876115. [DOI] [PubMed] [Google Scholar]
- Bakker E. P. Accumulation of thallous ions (Tl+) as a measure of the electrical potential difference across the cytoplasmic membrane of bacteria. Biochemistry. 1978 Jul 11;17(14):2899–2904. doi: 10.1021/bi00607a031. [DOI] [PubMed] [Google Scholar]
- Brading A. F. Sodium/sodium exchange in the smooth muscle of the guinea-pig taenia coli. J Physiol. 1975 Sep;251(1):79–105. doi: 10.1113/jphysiol.1975.sp011082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Widdicombe J. H. An estimate of sodium-potassium pump activity and the number of pump sites in the smooth muscle of the guinea-pig taenia coli, using (3H)ouabain. J Physiol. 1974 Apr;238(2):235–249. doi: 10.1113/jphysiol.1974.sp010521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten J. S., Blank M. Thallium activation of the (Na+--K+)-activated ATPase of rabbit kidney. Biochim Biophys Acta. 1968 Apr 24;159(1):160–166. doi: 10.1016/0005-2744(68)90254-4. [DOI] [PubMed] [Google Scholar]
- CSAPO I. A., KURIYAMA H. A. Effects of ions and drugs on cell membrane activity and tension in the postpartum rat myometrium. J Physiol. 1963 Mar;165:575–592. doi: 10.1113/jphysiol.1963.sp007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R. The distribution of chloride ions in the smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Apr;214(2):225–243. doi: 10.1113/jphysiol.1971.sp009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavieres J. D., Ellory J. C. Thallium and the sodium pump in human red cells. J Physiol. 1974 Nov;243(1):243–266. doi: 10.1113/jphysiol.1974.sp010752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damper P. D., Epstein W., Rosen B. P., Sorensen E. N. Thallous ion is accumulated by potassium transport systems in Escherichia coli. Biochemistry. 1979 Sep 18;18(19):4165–4169. doi: 10.1021/bi00586a018. [DOI] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve. Selective permeability to small cations. J Gen Physiol. 1973 Jun;61(6):669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson B. J., Daniel E. E. The effects of excitatory drugs on potassium fluxes in uterine smooth muscle. Can J Physiol Pharmacol. 1972 Jul;50(7):725–730. doi: 10.1139/y72-106. [DOI] [PubMed] [Google Scholar]
- Inturrisi C. E. Thallium activation of K+-activated phosphatases from beef brain. Biochim Biophys Acta. 1969 Apr;173(3):567–569. doi: 10.1016/0005-2736(69)90022-4. [DOI] [PubMed] [Google Scholar]
- Johns A., Paton D. M. Inhibitory effect of sodium pumping on spontaneous contractility of rabbit myometrium. Can J Physiol Pharmacol. 1974 Aug;52(4):786–790. doi: 10.1139/y74-103. [DOI] [PubMed] [Google Scholar]
- Landowne D. A comparison of radioactive thallium and potassium fluxes in the giant axon of the squid. J Physiol. 1975 Oct;252(1):79–96. doi: 10.1113/jphysiol.1975.sp011135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS L. J., MOORE R. D. The movement of thallium ions in muscle. J Gen Physiol. 1960 Mar;43:759–773. doi: 10.1085/jgp.43.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulskii I. A., Manninen V., Järnefelt J. Factors affecting the relative magnitudes of the ouabain-sensitive and the ouabain-insensitive fluxes of thallium ion in erythrocytes. Biochim Biophys Acta. 1978 Jan 19;506(2):233–241. doi: 10.1016/0005-2736(78)90394-2. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H. Ouabain-sensitive ion fluxes in the smooth muscle of the guinea-pig's taenia coli. J Physiol. 1977 Apr;266(2):235–254. doi: 10.1113/jphysiol.1977.sp011766. [DOI] [PMC free article] [PubMed] [Google Scholar]


