Abstract
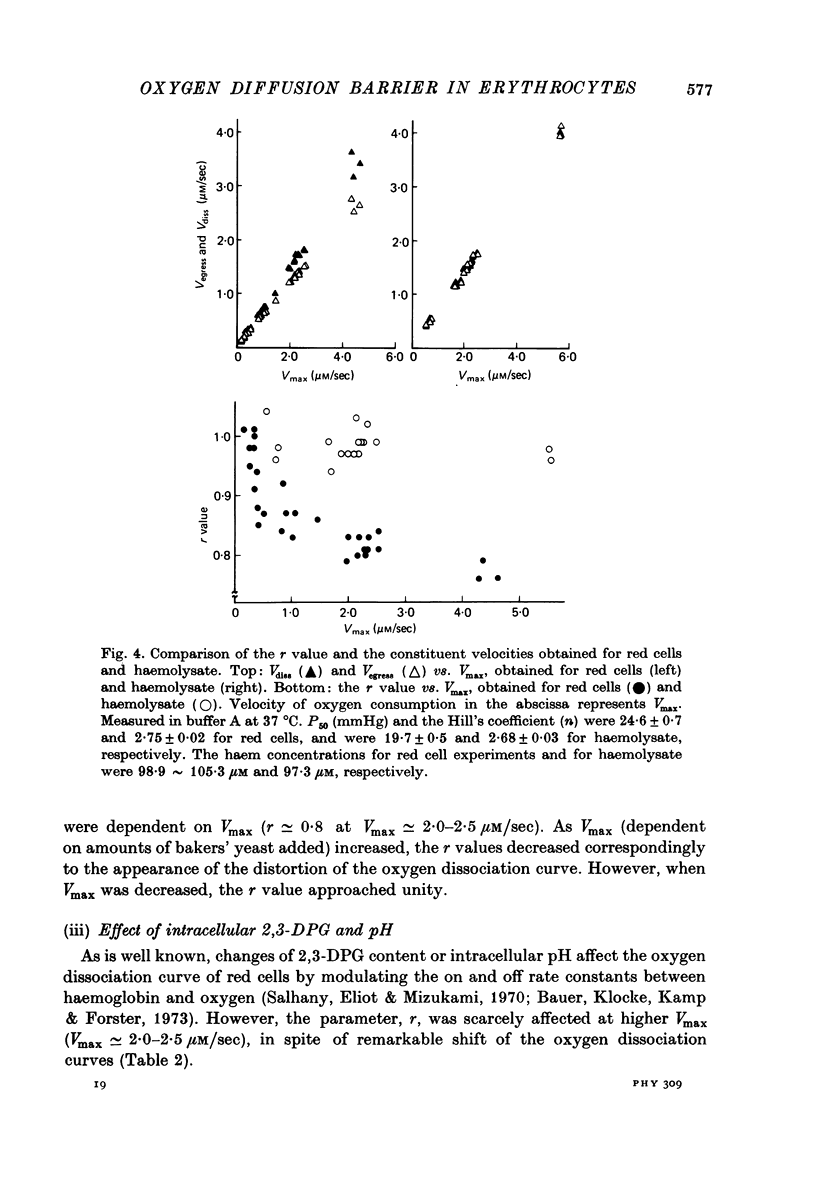
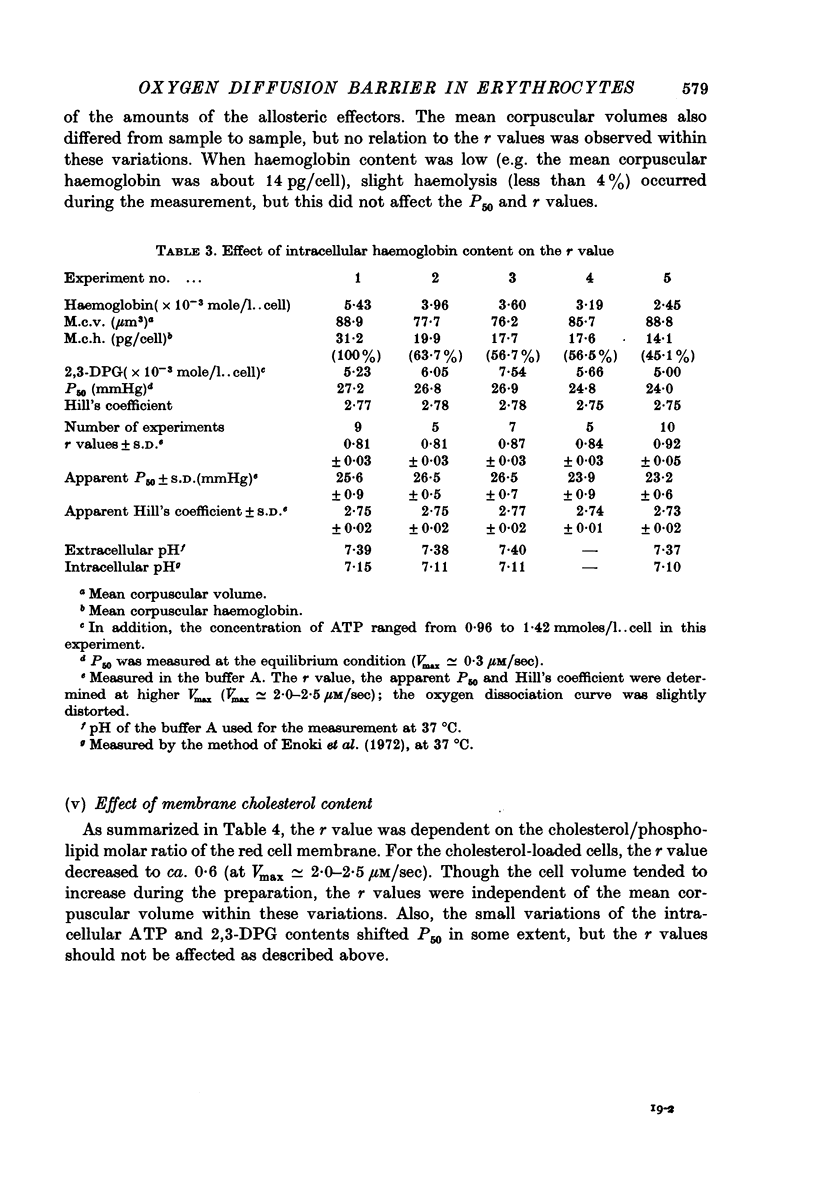
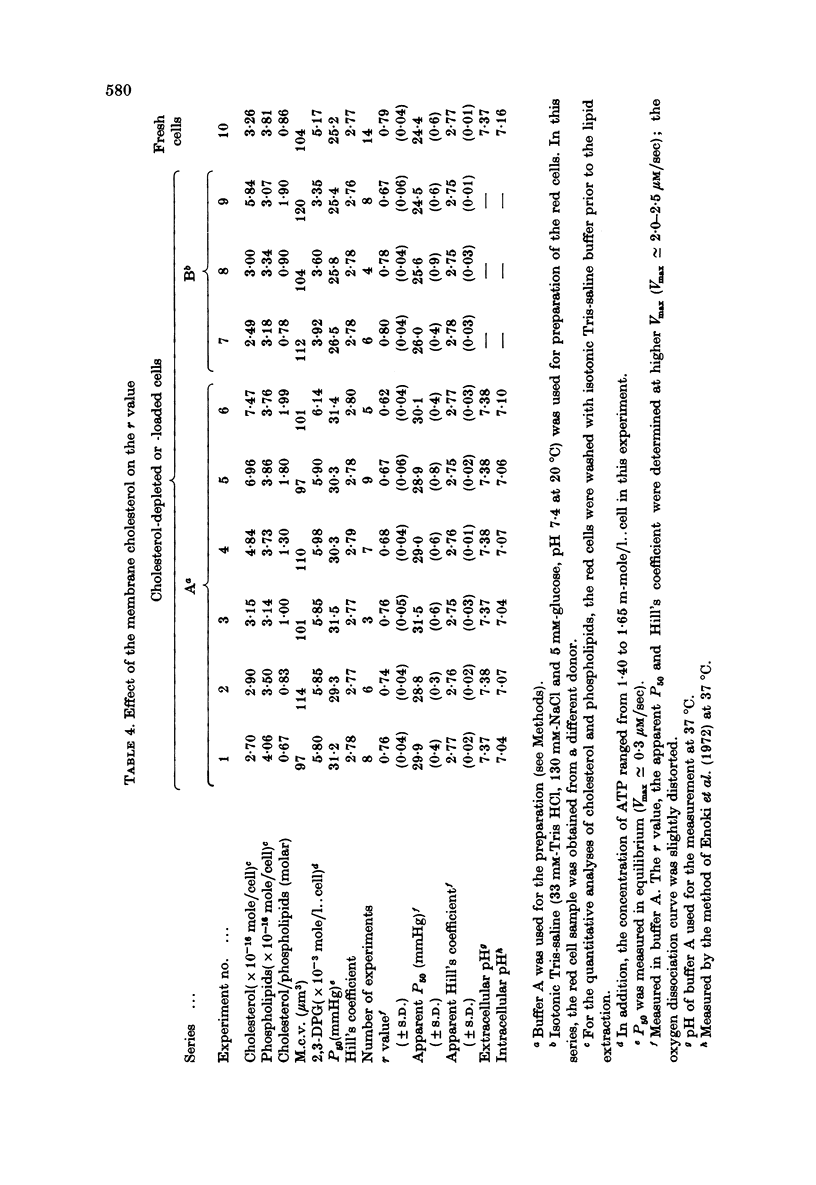
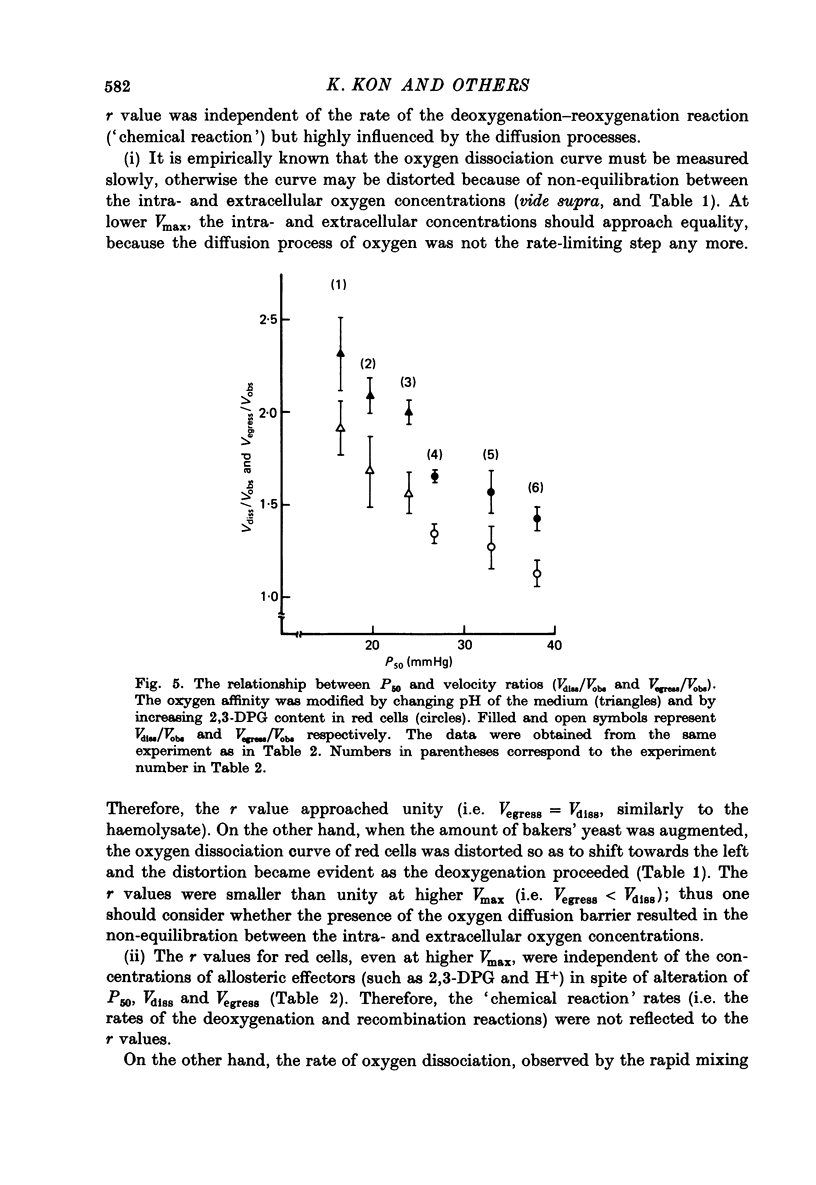
1. In order to study the kinetics os the oxygen egress from human red cells in the 50 sec-20 min time range, an apparatus for measuring the oxygen dissociation process was constructed, combining a spectrophotometer with an oxygen electrode of quick response. 2. Starting from air-saturated haemolysate or red cell suspensions, the velocity of oxygen dissociation from oxyhaemoglobin (Vdiss) and of oxygen disappearance in the medium (Vobs) after addition of bakers' yeast (consuming the dissolved oxygen at the velocity of Vconsump) were recorded. A parameter (r) was defined as the ratio of two velocities, Vegress (the velocity of oxygen egress into the medium) and Vdiss, r identical to Vegress/Vdiss = (Vconsump -Vobs)/Vdiss. Vcomsump could be calculated by the Michaelis-Menten equation as follows, Vconsump = Vmax [O2]/(Km + [O2]), where Vmax was the maximal velocity of oxygen consumption of bakers' yeast. 3. The r value was always 1.0 for the haemolysate, but it was less than 1.0 for the normal red cells. Further, the oxygen dissociation curve of red cells obtained at higher Vmax was distorted, due to the non-equilibration between intra- and extracellular oxygen concentrations. 4. The r value was (i) independent of the amounts of the allosteric effectors (2,3-diphosphoglycerate and H+) but (ii) dependent on the haemoglobin contents and (iii) dependent on the amounts of the membrane cholesterol. Therefore, the r value reflected only the process of the oxygen diffusion but not the "chemical reaction' rate. The "barrier' of the oxygen diffusion decreased at lower haemoglobin contents, but increased at higher cholesterol contents in the membrane.
Full text
PDF


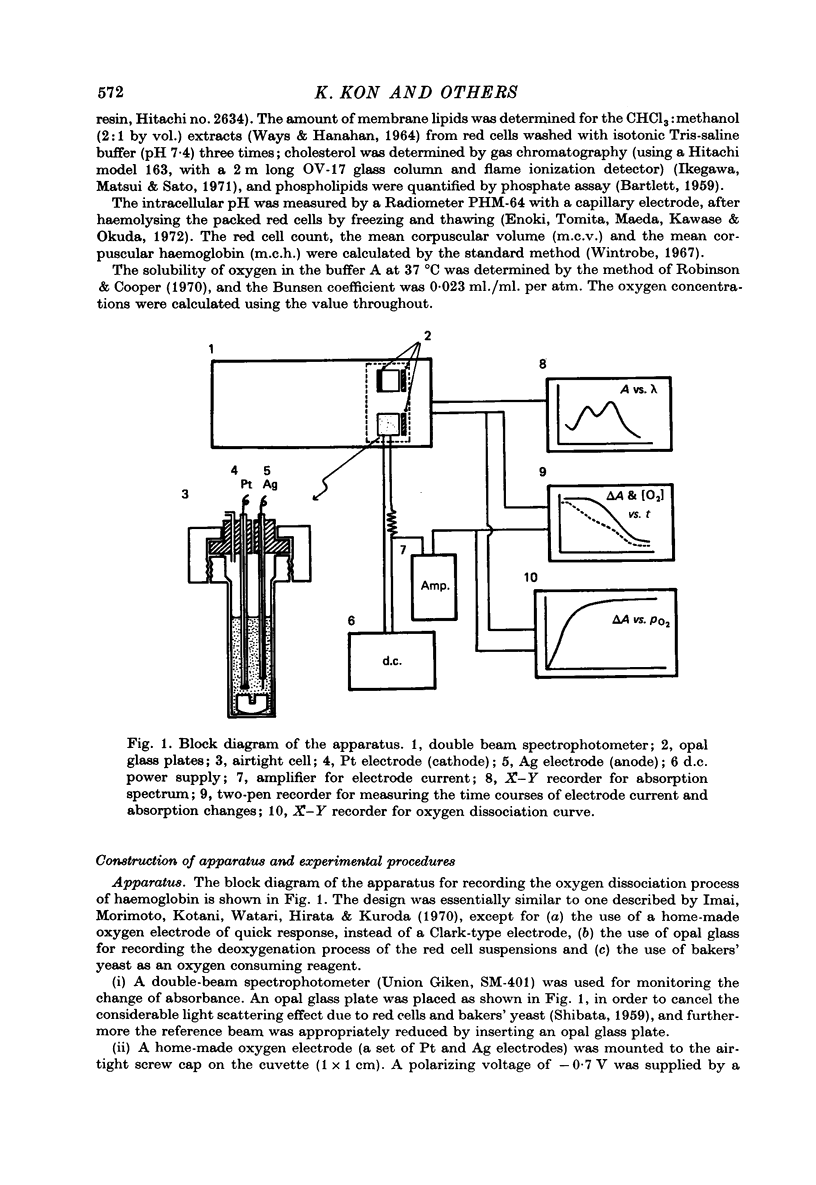
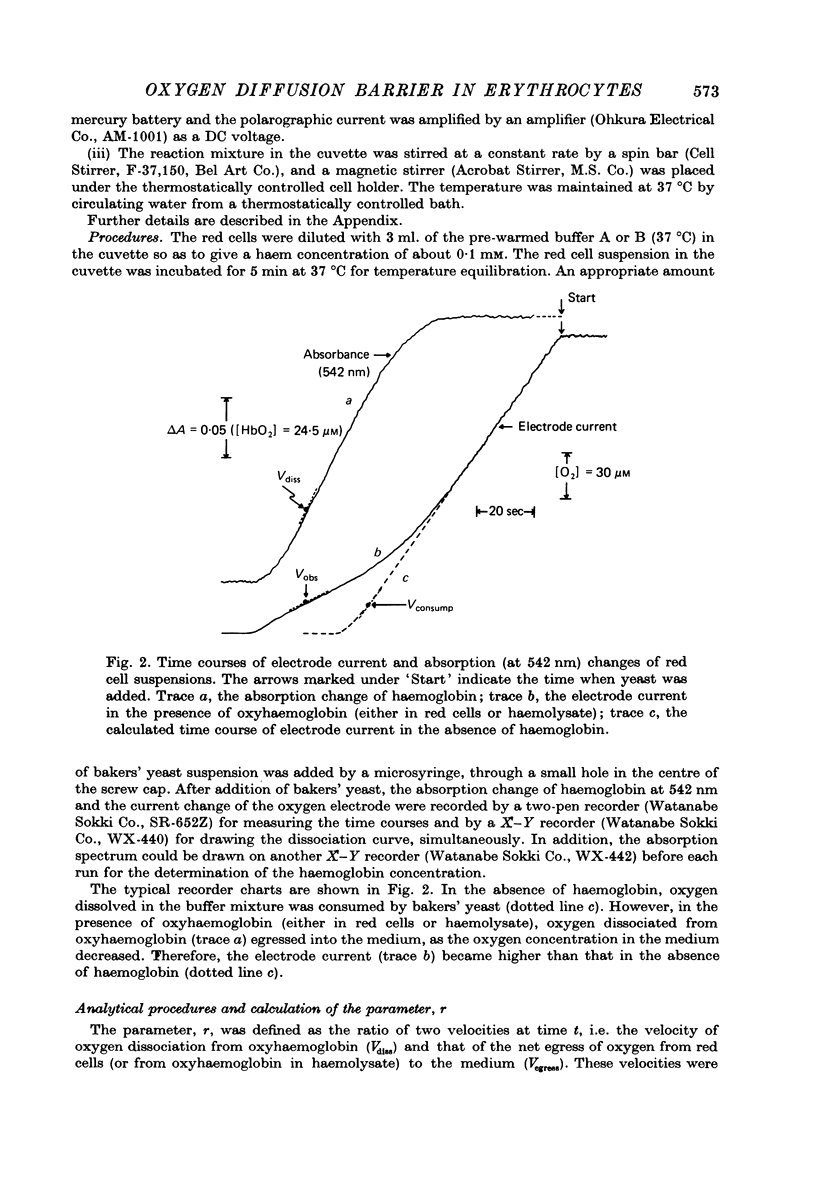

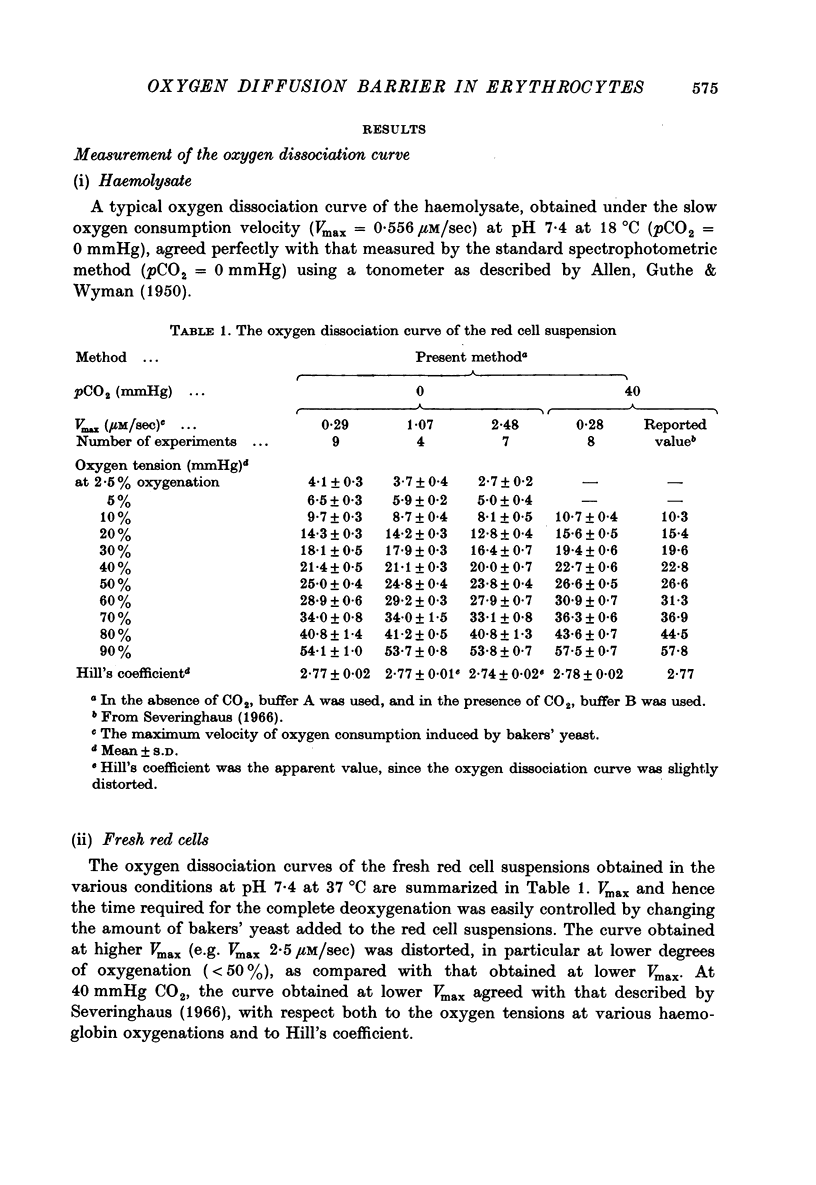
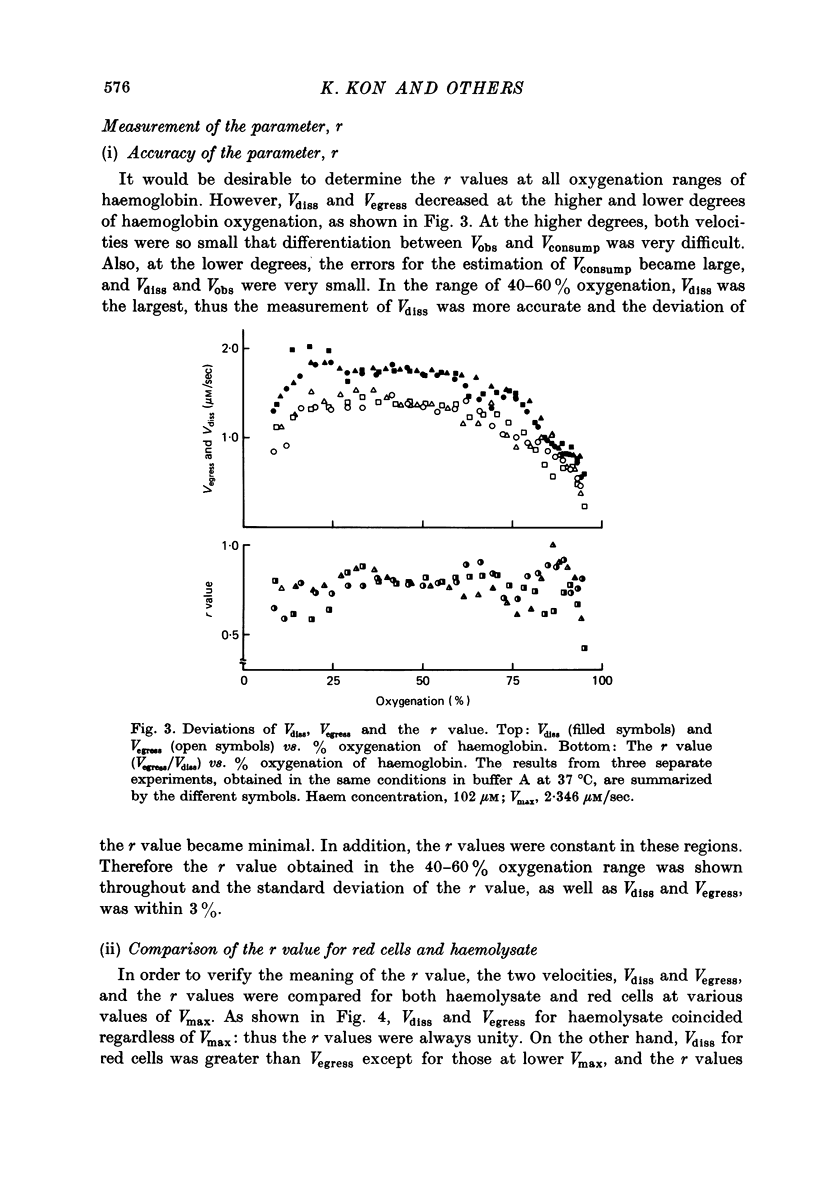




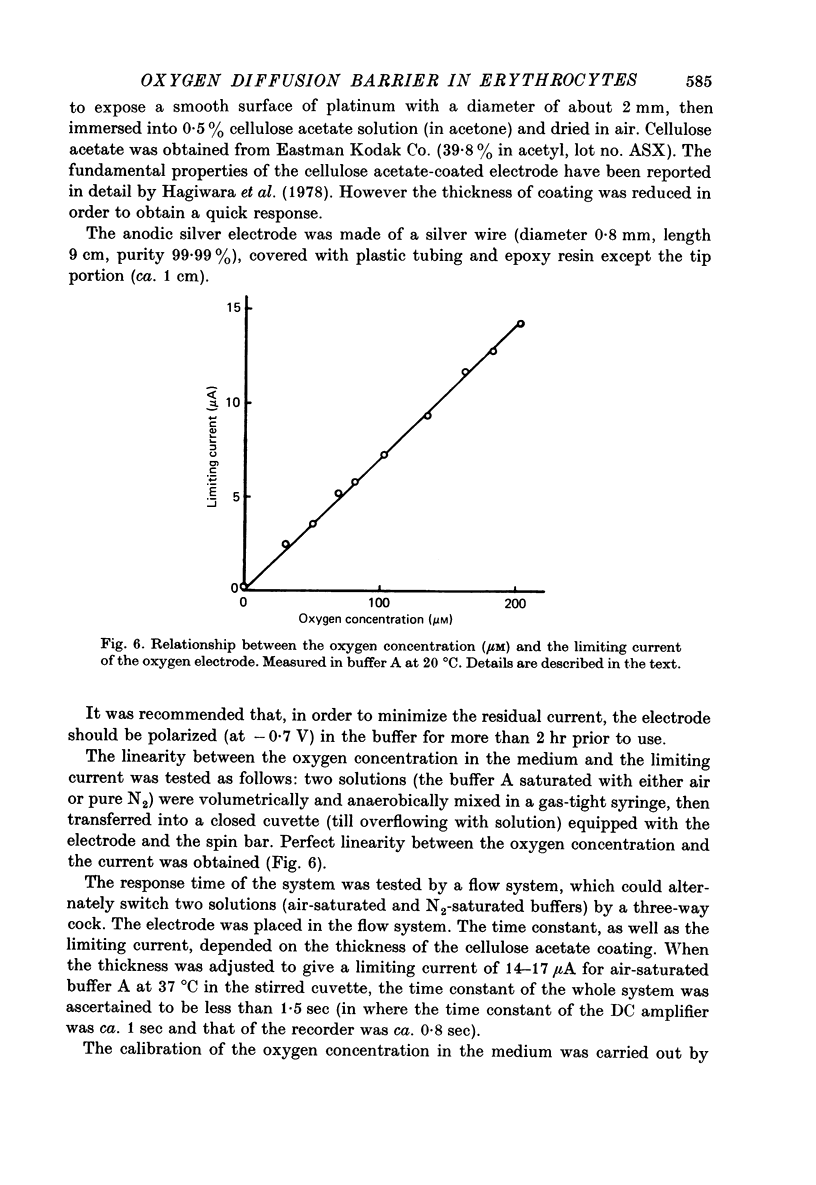
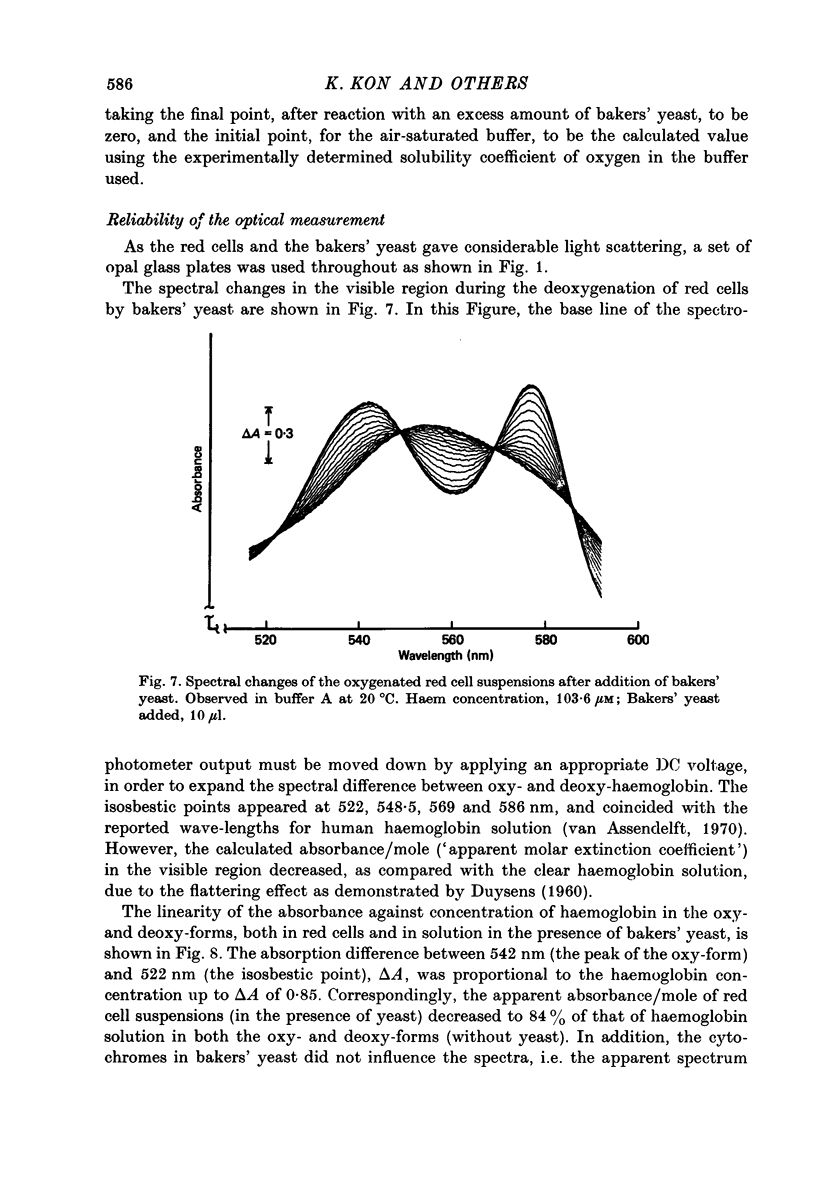
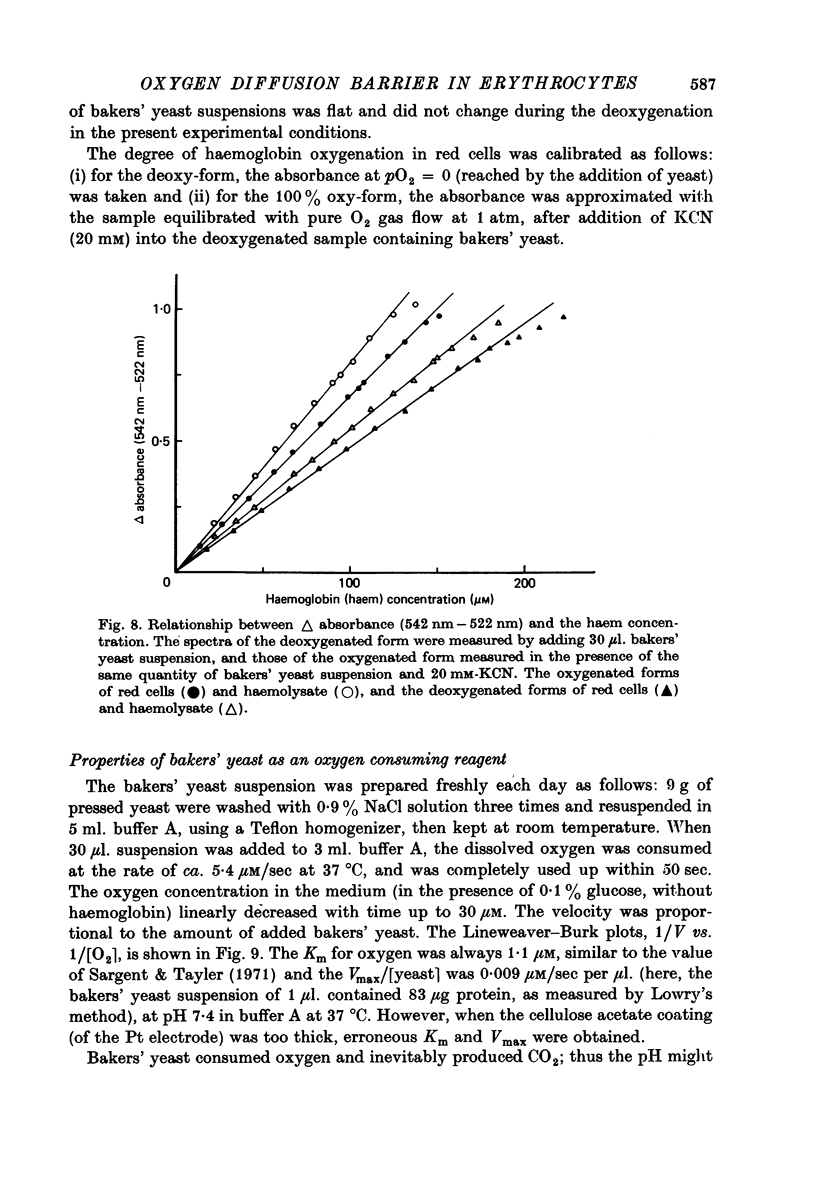
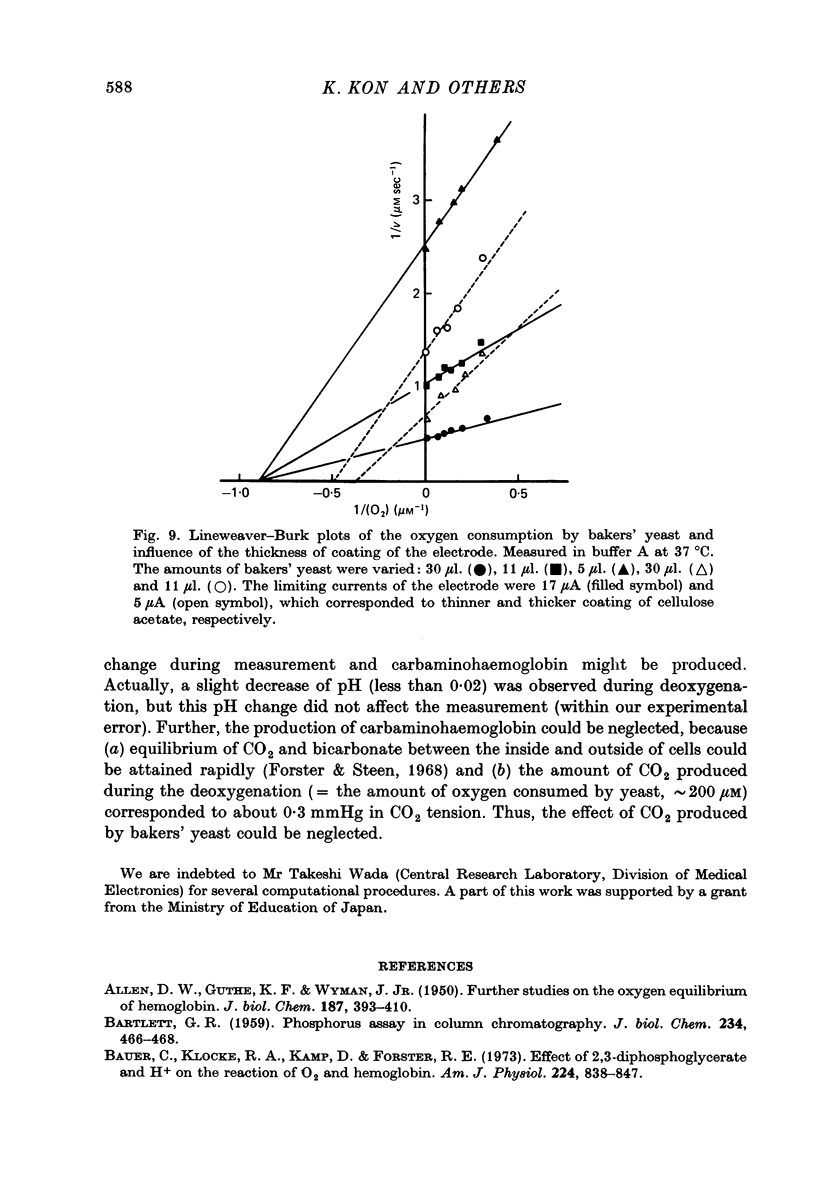


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN D. W., GUTHE K. F., WYMAN J., Jr Further studies on the oxygen equilibrium of hemoglobin. J Biol Chem. 1950 Nov;187(1):393–410. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bauer C., Klocke R. A., Kamp D., Forster R. E. Effect of 2,3-diphosphoglycerate and H+ on the raction of O 2 and hemoglobin. Am J Physiol. 1973 Apr;224(4):838–847. doi: 10.1152/ajplegacy.1973.224.4.838. [DOI] [PubMed] [Google Scholar]
- CARLSEN E., COMROE J. H., Jr The rate of uptake of carbon monoxide and of nitric oxide by normal human erythrocytes and experimentally produced spherocytes. J Gen Physiol. 1958 Sep 20;42(1):83–107. doi: 10.1085/jgp.42.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLMAN C. H., LONGMUIR I. S. A new method for registration of oxyhemoglobin dissociation curves. J Appl Physiol. 1963 Mar;18:420–423. doi: 10.1152/jappl.1963.18.2.420. [DOI] [PubMed] [Google Scholar]
- Coin J. T., Olson J. S. The rate of oxygen uptake by human red blood cells. J Biol Chem. 1979 Feb 25;254(4):1178–1190. [PubMed] [Google Scholar]
- Cooper R. A., Leslie M. H., Fischkoff S., Shinitzky M., Shattil S. J. Factors influencing the lipid composition and fluidity of red cell membranes in vitro: production of red cells possessing more than two cholesterols per phospholipid. Biochemistry. 1978 Jan 24;17(2):327–331. doi: 10.1021/bi00595a021. [DOI] [PubMed] [Google Scholar]
- DALZIEL K., O'BRIEN J. R. The kinetics of deoxygenation of human haemoglobin. Biochem J. 1961 Feb;78:236–245. doi: 10.1042/bj0780236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUYSENS L. N. The flattening of the absorption spectrum of suspensions, as compared to that of solutions. Biochim Biophys Acta. 1956 Jan;19(1):1–12. doi: 10.1016/0006-3002(56)90380-8. [DOI] [PubMed] [Google Scholar]
- Deuticke B., Duhm J., Dierkesmann R. Maximal elevation of 2,3-diphosphoglycerate concentrations in human erythrocytes: influence on glycolytic metabolism and intracellular pH. Pflugers Arch. 1971;326(1):15–34. doi: 10.1007/BF00586792. [DOI] [PubMed] [Google Scholar]
- Enoki Y., Tomita S., Maeda N., Kawase M., Okuda T. A simple method for determination of red cell intracellular pH. Nihon Seirigaku Zasshi. 1972 Nov;34(11):761–762. [PubMed] [Google Scholar]
- FORSTER R. E., ROUGHTON F. J., KREUZER F., BRISCOE W. A. Photocolorimetric determination of rate of uptake of CO and O2 by reduced human red cell suspensions at 37 degrees C. J Appl Physiol. 1957 Sep;11(2):260–268. doi: 10.1152/jappl.1957.11.2.260. [DOI] [PubMed] [Google Scholar]
- Fischer T. M., Stöhr-Lissen M., Schmid-Schönbein H. The red cell as a fluid droplet: tank tread-like motion of the human erythrocyte membrane in shear flow. Science. 1978 Nov 24;202(4370):894–896. doi: 10.1126/science.715448. [DOI] [PubMed] [Google Scholar]
- Fischkoff S., Vanderkooi J. M. Oxygen diffusion in biological and artificial membranes determined by the fluorochrome pyrene. J Gen Physiol. 1975 May;65(5):663–676. doi: 10.1085/jgp.65.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R. E., Steen J. B. Rate limiting processes in the Bohr shift in human red cells. J Physiol. 1968 Jun;196(3):541–562. doi: 10.1113/jphysiol.1968.sp008522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H., KREUZER F., MEDA E., ROUGHTON F. J. The kinetics of human haemoglobin in solution and in the red cell at 37 degrees C. J Physiol. 1955 Jul 28;129(1):65–89. doi: 10.1113/jphysiol.1955.sp005339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara B., Ishibashi F., Sasaki K., Kamigawara Y. Cellulose acetate coatings for the polarographic oxygen electrode. Anal Biochem. 1978 Jun 1;86(2):417–431. doi: 10.1016/0003-2697(78)90765-0. [DOI] [PubMed] [Google Scholar]
- Holland R. A., Forster R. E. The effect of size of red cells on the kinetics of their oxygen uptake. J Gen Physiol. 1966 Mar;49(4):727–742. doi: 10.1085/jgp.49.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikekawa N., Matsui M., Sato K. Simultaneous determination of cholesterol and cholesterol esters in serum by gas chromatography. Jpn J Exp Med. 1971 Jun;41(3):163–170. [PubMed] [Google Scholar]
- Imai K., Morimoto H., Kotani M., Watari H., Hirata W. Studies on the function of abnormal hemoglobins. I. An improved method for automatic measurement of the oxygen equilibrium curve of hemoglobin. Biochim Biophys Acta. 1970 Feb 17;200(2):189–196. doi: 10.1016/0005-2795(70)90163-7. [DOI] [PubMed] [Google Scholar]
- KREUZER F., YAHR W. Z. Influence of red cell membrane on diffusion of oxygen. J Appl Physiol. 1960 Nov;15:1117–1122. doi: 10.1152/jappl.1960.15.6.1117. [DOI] [PubMed] [Google Scholar]
- Kreuzer F. Facilitated diffusion of oxygen and its possible significance; a review. Respir Physiol. 1970 Apr;9(1):1–30. doi: 10.1016/0034-5687(70)90002-2. [DOI] [PubMed] [Google Scholar]
- Kutchai H. Role of the red cell membrane in oxygen uptake. Respir Physiol. 1975 Jan;23(1):121–132. doi: 10.1016/0034-5687(75)90076-6. [DOI] [PubMed] [Google Scholar]
- Kutchai H., Staub N. C. Steady-state, hemoglobin-facilitated O2 transport in human erythrocytes. J Gen Physiol. 1969 May;53(5):576–589. doi: 10.1085/jgp.53.5.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGMUIR I. S., ROUGHTON F. J. W. The diffusion coefficients of carbon monoxide and nitrogen in haemoglobin solutions. J Physiol. 1952 Oct;118(2):264–275. doi: 10.1113/jphysiol.1952.sp004791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson W. H., Jr, Holland R. A., Forster R. E. Effect of temperature on deoxygenation rate of human red cells. J Appl Physiol. 1965 Sep;20(5):912–918. doi: 10.1152/jappl.1965.20.5.912. [DOI] [PubMed] [Google Scholar]
- Lawson W. H., Jr Interrelation of pH, temperature, and oxygen on deoxygenation rate of red cells. J Appl Physiol. 1966 May;21(3):905–914. doi: 10.1152/jappl.1966.21.3.905. [DOI] [PubMed] [Google Scholar]
- MOCHIZUKI M., FUKUOKA J. The diffusion of oxygen inside the red cell. Jpn J Physiol. 1958 Sep 15;8(3):206–224. doi: 10.2170/jjphysiol.8.206. [DOI] [PubMed] [Google Scholar]
- Maeda N., Chang H., Benesch R., Benesch R. E. A simple enzymatic method for the determination of 2,3-diphosphoglycerate in small amounts of blood. N Engl J Med. 1971 Jun 3;284(22):1239–1242. doi: 10.1056/NEJM197106032842204. [DOI] [PubMed] [Google Scholar]
- NICOLSON P., ROUGHTON F. J. W. A theoretical study of the influence of diffusion and chemical reaction velocity on the rate of exchange of carbon monoxide and oxygen between the red blood corpuscle and the surrounding fluid. Proc R Soc Lond B Biol Sci. 1951 Jun;138(891):241–264. doi: 10.1098/rspb.1951.0020. [DOI] [PubMed] [Google Scholar]
- ROUGHTON F. J., FORSTER R. E., CANDER L. Rate at which carbon monoxide replaces oxygen from combination with human hemoglobin in solution and in the red cell. J Appl Physiol. 1957 Sep;11(2):269–276. doi: 10.1152/jappl.1957.11.2.269. [DOI] [PubMed] [Google Scholar]
- Robinson J., Cooper J. M. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970 Feb;33(2):390–399. doi: 10.1016/0003-2697(70)90310-6. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Eliot R. S., Mizukami H. The effects of 2,3-diphosphoglycerate on the kinetics of deoxygenation of human hemoglobin. Biochem Biophys Res Commun. 1970;39(6):1052–1057. doi: 10.1016/0006-291x(70)90665-0. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Keitt A. S., Eliot R. S. The rate of deoxygenation of red blood cells: Effect of intracellular 2,3-diphosphoglycerate and pH. FEBS Lett. 1971 Sep 1;16(4):257–261. doi: 10.1016/0014-5793(71)80364-2. [DOI] [PubMed] [Google Scholar]
- Sargent D. F., Taylor C. P. A stable long-term differentiator and its use in the automatic recording of enzyme kinetics. Anal Biochem. 1971 Aug;42(2):446–454. doi: 10.1016/0003-2697(71)90058-3. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein H. Microrheology of erythrocytes, blood viscosity, and the distribution of blood flow in the microcirculation. Int Rev Physiol. 1976;9:1–62. [PubMed] [Google Scholar]
- Severinghaus J. W. Blood gas calculator. J Appl Physiol. 1966 May;21(3):1108–1116. doi: 10.1152/jappl.1966.21.3.1108. [DOI] [PubMed] [Google Scholar]
- Shiga T., Maeda N., Suda T., Kon K., Sekiya M., Oka S. Rheological and kinetic dysfunctions of the cholesterol-loaded, human erythrocytes. Biorheology. 1979;16(4-5):363–369. doi: 10.3233/bir-1979-164-511. [DOI] [PubMed] [Google Scholar]
- Sirs J. A. The egress of oxygen from sheep erythrocytes after mixing with sodium dithionite. Biochim Biophys Acta. 1966 Sep 5;126(1):28–36. doi: 10.1016/0926-6585(66)90033-1. [DOI] [PubMed] [Google Scholar]
- Sirs J. A. The egress of oxygen from sheep erythrocytes. Bibl Laeger. 1966 Mar 14;112(3):538–549. doi: 10.1016/0926-6585(66)90257-3. [DOI] [PubMed] [Google Scholar]
- Sirs J. A. The respiratory efficiency and flexibility of erythrocytes stored in acid-citrate-dextrose solution. J Physiol. 1969 Jul;203(1):93–109. doi: 10.1113/jphysiol.1969.sp008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., Maeda N., Sekiya M., Matsuoka K., Tokita R., Shiga T. Influences of membrane cholesterol on the human red cell properties. Med J Osaka Univ. 1978 Sep;29(1-2):21–28. [PubMed] [Google Scholar]
- THEWS G. DIE THEORETISCHEN GRUNDLAGEN DER SAUERSTOFFAUFNAHME IN DER LUNGE. Ergeb Physiol. 1963;53:42–107. doi: 10.1007/BF02259178. [DOI] [PubMed] [Google Scholar]
- Ways P., Hanahan D. J. Characterization and quantification of red cell lipids in normal man. J Lipid Res. 1964 Jul;5(3):318–328. [PubMed] [Google Scholar]
- van KAMPEN E., ZIJLSTRA W. G. Standardization of hemoglobinometry. II. The hemiglobincyanide method. Clin Chim Acta. 1961 Jul;6:538–544. doi: 10.1016/0009-8981(61)90145-0. [DOI] [PubMed] [Google Scholar]


