Abstract
The commonly used antiviral drugs acyclovir (ACV) and penciclovir (PCV) possess similarly potent antiviral activities in vivo against herpes simplex virus (HSV). Assay methods for sensitivity to ACV are not necessarily transferable to PCV, even though the two drugs have similar in vivo potencies and mechanisms of action. We determined by plaque reduction assay the relative activities of ACV and PCV against five laboratory-adapted strains of HSV types 1 and 2 (including sensitive and resistant strains) in seven human cell lines and one nonhuman primate cell line. Seven characteristics were used to evaluate the cell lines. All cell lines were similar in their plating efficiencies and abilities to discriminate between sensitive and resistant HSV isolates. Vero and MRC-5 cells yielded the most discordant 50% inhibitory concentrations (IC50s) for the two HSV types, while Vero and WI-38 VA-13 cells yielded large differences in the IC50s of ACV and PCV. The limited life spans and poor plaque morphologies of the fibroblast lines were undesirable characteristics. Among the transformed cell lines producing well-defined plaques, A549 cells provided the best concordance between IC50s for the two HSV types and two antiherpes drugs. Comparison experiments with a yield reduction format indicated that the use of assays of this type might allow some of the cell-specific properties observed in plaque reduction assays to be avoided.
Antiviral susceptibility testing of herpes simplex virus (HSV) clinical isolates is important because of the frequency of infection of immunocompromised hosts with resistant HSV strains and the increasing worldwide use of antiviral analog therapy. The National Committee for Clinical Laboratory Standards (NCCLS) has proposed a standard method (18) for in vitro determination of HSV susceptibility to two antivirals, acyclovir (ACV) and foscarnet (phosphonoformic acid [FOS]). For other prescribed antiviral drugs, such as penciclovir (PCV) and its prodrug famciclovir, the standard may need to be modified. The plaque reduction assay (PRA) provided in the NCCLS standard is the most commonly reported method for evaluations of the susceptibilities of clinical isolates to ACV and the technique to which new methods are compared (16, 18, 22, 24, 26, 28, 30). ACV resistance of HSV measured by PRA correlates with isolates from clinical infections unresponsive to ACV therapy; thus, the PRA is biologically relevant (25).
Testing of the susceptibilities of HSV isolates to ACV has typically been performed in Vero (African green monkey kidney), MRC-5, or HEL (diploid human embryonic lung) cells. A priori, lines derived from human cells would be preferred for a standard assay, since current and future drugs are developed and optimized for activity in the human system. Vero cells do represent a continuous cell line that readily forms distinct plaques upon infection with HSV, but they are nonhuman in origin. Conversely, MRC-5 and HEL cells, which are human in origin and which have the appropriate metabolism (11, 32), represent primary cell lines with limited life spans and inabilities to form distinct plaques upon HSV infection.
Pursuant to broadening the clinical application of PRA to PCV, we undertook a careful analysis of potential host cells, the most critical element of the assay. Major modifications from the standard assay for ACV might not be expected, since PCV and ACV share nearly identical mechanisms of action and in vivo efficacies and since ACV-resistant strains are nearly all cross resistant to PCV (1-3, 9-11, 13, 14, 23, 29, 32, 33; for reviews, see references 2, 4, and 6). However, three observations suggested that cell-dependent differences in HSV susceptibility to PCV compared to that to ACV as determined by PRA could be significant. First, the range of 50% inhibitory concentrations (IC50s) for identical HSV strains can be more than 1 log of the ACV or PCV concentration as a function of the host cells used (4, 17). Second, the IC50s for HSV type 1 (HSV-1) strains can be many fold lower than those for HSV-2 strains in some cells. The IC50 for resistant HSV-1 strain can be equivalent to or less than that for a susceptible HSV-2 strain, and thus, a different resistance cutoff for types 1 and 2 could be needed (21; unpublished observations). Third, in a few reports of studies with some cells, the relative potency of PCV is very different from that of ACV, despite the drugs' similarities, which strictly limits the value of drug-to-drug susceptibility comparisons (4). Susceptibility tests with both drugs with such cells might suggest a phenotypic resistance to ACV and sensitivity to PCV, or the reverse, when in fact the isolate is susceptible to both PCV and ACV. A host cell line for use in PRA in which PCV and ACV have similar activities against both HSV-1 and HSV-2 would be very useful.
In view of the issues outlined above, we determined the susceptibilities of phenotypically and genotypically characterized drug-resistant and drug-sensitive HSV standard strains to PCV and ACV by a standard PRA with eight cell lines. Additionally, we used a yield reduction susceptibility test with these cell lines to validate the PRA results. The yield test is not dependent upon the counting of plaques in the presence of the drug, and so the result is not subject to variations in plaque morphology or size, which have critical effects on the results of PRA. Furthermore, all combinations of virus and cells were also tested with FOS, an antiviral pyrophosphate analogue which does not require cellular phosphorylation to be active against HSV and which is already included in the NCCLS proposed standard (18).
MATERIALS AND METHODS
Viruses and cell lines.
Table 1 lists the HSV strains and cell lines used in this study. HSV-2 SB5 (ATCC VR-2546) is a plaque-purified derivative of HSV-2 strain 333. HSV-2 strain 333 was generously provided as a stock after less than six passages in human diploid fibroblasts after clinical isolation by P. A. Schaffer (Beth Israel-Deaconess Medical Center, Boston, Mass.). HSV-1 SC16, SC16S1, and DM21 were generously provided by S. Safrin (Gilead Sciences, Inc., Foster City, Calif.). All cell lines were obtained as seed stocks from the American Type Culture Collection (ATCC; Manassas, Va.), cultured as recommended by ATCC, and used at passage 20 or less.
TABLE 1.
Virus strains and host cell lines
| Virus or cell line (ATCC no.) | Description (reference) | Phenotype |
|---|---|---|
| Viruses | ||
| HSV-2 SB5 | Wild-type strain of HSV-2, plaque purified from HSV-2 333PAS | ACVs PCVs |
| HSV-2 333 | HSV-2 333PAS clinical isolate, low passage, all passages made in human cells | ACVs PCVs |
| HSV-1 SC16 | Wild-type strain of HSV-1 (8) | ACVs PCVs |
| HSV-1 DM21 | HSV-1 strain SC16 with a deletion in TK (12) | TK− ACVr PCVr |
| HSV-1 SC16S1 | HSV-1 strain SC16 with an altered TK (8) | TKa ACVr PCVr |
| Cell lines | ||
| Vero (CCL-81) | Monkey kidney epithelial cells, transformed line | |
| A549 (CCL-185) | Human lung carcinoma epithelial cells, transformed line | |
| HEL 299 (CCL-137) | Diploid human embryonic lung cells, fibroblasts | |
| WISH (CCL-25) | Human amnion epithelial cells, transformed line | |
| SCC-25 (CRL-1628) | Human tongue squamous cell carcinoma, transformed line | |
| WI-38 VA-13 (CCL-75.1) | Simian virus 40-transformed WI-38 cells, epithelial cells | |
| MRC-5 (CCL-171) | Diploid human embryonic lung cells, fibroblasts | |
| Hs68 (CRL-1635) | Diploid human foreskin cells, fibroblasts |
Antiviral compounds.
PCV [9-(4-hydroxy-3-hydroxymethlybut-1-yl) guanine], obtained from the SmithKline Beecham compound bank (compound no. BRL-39123, batch 10), was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mg/ml. ACV [9-(2-hydroxyethoxymethyl) guanine; catalog no. A-4669; Sigma Chemical Co., St. Louis, Mo.] was dissolved in DMSO at a concentration of 10 mg/ml. FOS (Sigma Chemical Co.) was dissolved in water at a concentration of 40 mg/ml. All compounds were stored as single-use aliquots at −20°C. Dilutions were made in 2× Dulbecco's modified Eagle medium (DMEM; Life Technologies, Gaithersburg, Md.) supplemented with 10% heat-inactivated fetal calf serum (FCS) immediately before use.
PRA.
Confluent cell monolayers prepared with 2 × 105 to 4 × 105 cells/well in 12-well plates were infected with 100 PFU of HSV/well in 0.5 ml of Hanks balanced salt solution (HBSS) at 37°C in 5% CO2 for 1 h. Following virus adsorption, the inoculum was removed and 2 ml of overlay medium containing DMEM, 5% (vol/vol) heat-inactivated FCS, 0.4% (wt/vol) SeaPlaque agarose (FMC BioProducts, Rockland, Maine), and the appropriate concentration of ACV, PCV, or FOS was added to each well. Fourfold dilutions of ACV or PCV were tested at final concentrations ranging from 100 to 0.09 μg/ml for drug-resistant strains SC16S1 and DM21 or from 25 to 0.02 μg/ml for the wild-type virus strains. For all viruses, fourfold dilutions of FOS were tested, at final concentrations ranging from 400 to 1.56 μg/ml. Virus-infected control wells and uninfected control wells without antiviral compound were included in each assay. Susceptibility test plates were incubated for 48 h and fixed by overlaying them with 1.0 ml of 10% formaldehyde for 1 h at room temperature, the agar plug was aspirated, and infected monolayers were stained with 0.5% crystal violet prepared in 70% methanol. The viral plaques were counted, and IC50s were calculated by the method of Kärber (20).
Plating efficiency determination.
Confluent monolayers of all cell lines were adsorbed with 100 PFU/well, as determined in Vero cells, for 1 h at 37°C. Subsequently, the inoculum was removed and DMEM agarose overlay medium was added. Forty-eight hours later, the plates were fixed and stained and the plaques were counted. Plating efficiencies were calculated from the number of plaques produced by a virus suspension on human cells divided by the number of plaques produced by the same virus suspension on Vero cell monolayers.
YRA.
Confluent cell monolayers in 12-well plates (2 × 105 to 4 × 105 cells/well) were infected with HSV at a multiplicity of infection of 0.3 in 500 μl of HBSS at 37°C for 1 h. Following virus adsorption, the inoculum was removed, the cell monolayer was washed twice with 2.0 ml of HBSS, and the cultures were exposed in duplicate to 1.0 ml of a fourfold serial dilution of the test compounds prepared in DMEM plus 10% FCS. Seventy-two hours postinfection, the cells were scraped into the medium and disrupted by three freeze-thaw cycles. Cell-free HSV titers were determined in duplicate by plaque assay titration of the lysates in Vero cell monolayers, as described above for the PRA, but without antiviral compounds in the overlay. The end point for the yield reduction assay (YRA) was the concentration which reduced the virus yield by 50% relative to the yield from untreated control cultures. The YRA IC50 were calculated by the method of Kärber (20).
Statistical analysis.
The 95% confidence intervals for the difference between geometric mean IC50s were constructed for all comparisons of interest. Differences which fell outside of these intervals were considered statistically significant (P < 0.05).
RESULTS
Plating efficiency evaluation.
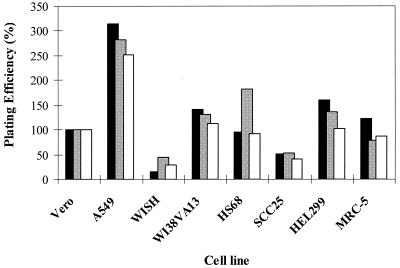
Since a cell line for antiviral assays must first be susceptible to HSV infection, the plating efficiencies of wild-type HSV strains in the eight candidate diploid or continuous cell lines were determined. The number of plaques formed on monolayers after infection with 100 PFU of HSV (titers were determined in Vero cells) was evaluated. Plating efficiency is defined as the number of plaques formed in the cell line tested divided by the number of plaques formed in Vero cells and multiplied by 100%. Efficiencies of plating for HSV-1 strain SC16 and HSV-2 strains SB5 and 333 are presented in Fig. 1. The plating efficiencies for all three viruses in any one cell line were similar, but there were considerable variations between cell lines. The plating efficiencies in A549 cells were 250 to 320%, but they were 50% or less in WISH and SCC-25 cells. The other cell lines plated HSV with efficiencies close to the efficiency achieved with Vero cells (100%). The plating efficiencies obtained with ACV-resistant strains SC16S1 and DM21 were similar to those obtained with sensitive viruses (data not shown). All eight cell lines supported HSV plating sufficiently for PRAs.
FIG. 1.
Relative susceptibilities of cell lines to HSV infection. The efficiencies of plating of HSV-2 SB5 (dark bars), HSV-2 333 (light bars), and HSV-1 SC16 (open bars) on the cell lines were determined. Confluent monolayers were infected in triplicate with the same dilution series of each virus. Two days later the plates were fixed, stained, and scored for plaque numbers. The number of plaques formed relative to the number formed on monolayers of Vero cells was calculated as a percentage.
PRAs.
PRAs were performed to compare the susceptibilities of five standard virus strains to ACV, PCV, and FOS (Table 2). The relative susceptibilities to PCV or ACV of wild-type virus strains (HSV-2 SB5 and 333 and HSV-1 SC16) varied with the host cell. The mean IC50s of both nucleoside analogs were significantly lower for HSV-1 SC16 (P < 0.05) than for the HSV-2 strains in all cells other than A549 and Hs68. Additionally, both type 1 and type 2 wild-type viruses were significantly more susceptible, on average, to ACV than to PCV in Vero cells and, albeit to a lesser degree, in MRC-5 and HEL 299 cells (P < 0.05). In contrast, the same virus strains were significantly less susceptible to ACV than to PCV in the WI-38 VA-13 and WISH cell lines (P < 0.05). The difference in wild-type HSV susceptibility to ACV compared to that to PCV was not significant in the A549, SCC-25, and Hs68 cell lines. The cell line also strongly affected the activity of the pyrophosphate analog FOS (Table 2). In several lines, FOS IC50s of >100 μg/ml were obtained for susceptible strains SC16 and 333. The FOS IC50s were significantly lower in Vero and WISH cells than in A549, Hs68, and WI-38 VA-13 cells (P < 0.05).
TABLE 2.
IC50s for five HSV strains determined by PRA in eight cell lines
| Drug and virus | IC50 (μg/ml) in the following cell linea
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Vero | A549 | HEL 299 | WISH | SCC-25 | WI-38 VA-13 | Hs68 | MRC-5 | |
| ACV | ||||||||
| SB5 | 0.17 ± 0.03 | 1.79 ± 0.35 | 0.21 ± 0.01 | 1.74 ± 0.71 | 0.65 ± 0.18 | 5.13 ± 1.36 | 0.36 ± 0.14 | 0.52 ± 0.18 |
| 333 | 0.35 ± 0.02 | 2.42 ± 1.06 | 0.18 ± 0.04 | 1.32 ± 0.23 | 0.71 ± 0.07 | 3.63 ± 0.28 | 0.51 ± 0.15 | 0.72 ± 0.33 |
| SC16 | 0.04 ± 0.00 | 3.22 ± 0.75 | 0.13 ± 0.03 | 0.54 ± 0.03 | 0.35 ± 0.09 | 4.19 ± 1.23 | 0.24 ± 0.04 | 0.28 ± 0.08 |
| SC16S1 | 13.38 ± 2.05 | >100 | 12.2 ± 1.36 | >100 | >100 | >100 | 30.59 ± 3.23 | 108 ± 75.7 |
| DM 21 | 11.63 ± 4.32 | >100 | 38.98 ± 9.01 | >100 | >100 | >100 | 88.22 ± 29.5 | 81.83 ± 19.4 |
| PCV | ||||||||
| SB5 | 1.43 ± 0.61 | 3.13 ± 0.48 | 0.48 ± 0.24 | 0.46 ± 0.16 | 0.34 ± 0.06 | 1.35 ± 0.39 | 0.56 ± 0.27 | 1.83 ± 0.64 |
| 333 | 2.87 ± 0.25 | 3.01 ± 1.41 | 0.69 ± 0.11 | 0.62 ± 0.14 | 0.52 ± 0.06 | 0.85 ± 0.1 | 0.72 ± 0.26 | 1.52 ± 0.18 |
| SC16 | 0.34 ± 0.03 | 1.76 ± 0.24 | 0.16 ± 0.04 | 0.19 ± 0.16 | 0.17 ± 0.04 | 0.38 ± 0.04 | 0.35 ± 0.12 | 0.40 ± 0.11 |
| SC16S1 | 11.6 ± 1.33 | >100 | 5.26 ± 1.28 | 40.79 ± 7.79 | 17.87 ± 10 | 54.82 ± 17.14 | 8.25 ± 4.09 | 45.96 ± 42.3 |
| DM 21 | 32.62 ± 3.81 | >100 | 37.04 ± 5.09 | >100 | >100 | >100 | 30.56 ± 24.4 | 54.82 ± 17.0 |
| FOS | ||||||||
| SB5 | 21.94 ± 5.45 | 81.81 ± 11.65 | 31.27 ± 2.58 | 27.57 ± 9.06 | 25.41 ± 1.89 | 55.41 ± 1.61 | >100 | 75.61 ± 27.15 |
| 333 | 34.93 ± 3.28 | 191.75 ± 86.94 | 39.81 ± 4.5 | 27.18 ± 7.23 | 45.64 ± 6.32 | 70.94 ± 8.8 | 58.62 ± 12.8 | 55.79 ± 24.25 |
| SC16 | 26.17 ± 4.94 | 22.54 ± 2.8 | 42.88 ± 2.12 | 61.98 ± 34.84 | 78.37 ± 10.11 | >100 | >100 | 90.89 ± 86.89 |
| SC16S1 | 32.8 ± 3.54 | >100 | 38.01 ± 4.16 | 40.85 ± 16.14 | 85.35 ± 29.47 | 95.09 ± 17.05 | >100 | >100 |
| DM 21 | 31.03 ± 12.08 | >100 | >100 | 69.33 ± 38.74 | 55.54 ± 6.49 | >100 | 74.35 ± 2.08 | 99.33 ± 7.54 |
Each value represents the mean ± standard deviation IC50 for three assays. Each assay was done with duplicate wells over a range of five drug dilutions.
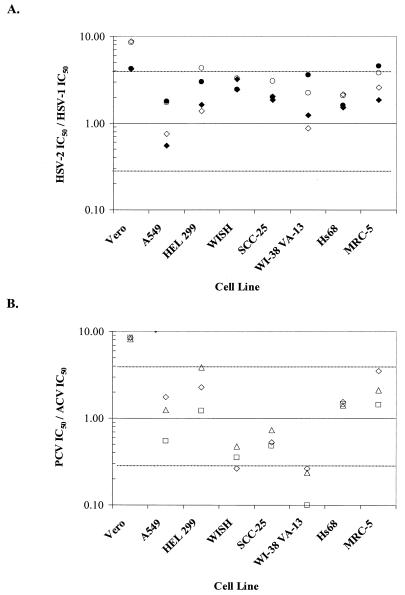
To visualize the cell-dependent differences in IC50s between type 1 and type 2 HSV isolates, the ratio of the IC50 for HSV-2 to that for HSV-1 was calculated for each cell line (Fig. 2A). An IC50 ratio of 1.0 indicates identical susceptibilities of both HSV types in a cell line. In Fig. 2 and 3,±1 dilution reference lines (at ratios of 4.0 and 0.25) are used as general guides to the magnitudes of the differences. The statistical 95% confidential intervals are smaller, 0.3 to 3.1 or even 0.6 to 1.8 for global assessments (e.g., the IC50 for HSV-1 is less than the IC50 for HSV-2 in all cell lines for both drugs). The HSV-2 strains appeared to be less sensitive than HSV-1 SC16 to either drug in most lines, but particularly in Vero cells. The ratios were close to 1.0 for A549 and Hs68 cells with both ACV and PCV and for HEL 299 and WI-38 VA-13 cells with ACV only. All of the other ratios except those under one condition each in HEL-299 and MRC-5 cells fell between values of 0.25 and 4.0, so the IC50s were within 1 drug dilution (fourfold) of each other.
FIG. 2.
Effect of cell line on HSV type-specific susceptibility to ACV and PCV by PRA. (A) Ratios of the IC50 for HSV-2 333 to the IC50 for HSV-1 SC16 (open symbols) and the IC50 for HSV-2 SB5 to the IC50 for HSV-1 SC16 (filled symbols) for both ACV (diamonds) and PCV (circles) for each cell line tested. (B) Ratio of PCV IC50 to ACV IC50 for the three standard virus strains (HSV-1 SC16, squares; HSV-2 333, triangles; and HSV-2 SB5, diamonds ) in each cell line tested. The dashed lines represent IC50 ratios that differ by ±1 drug dilution (fourfold).
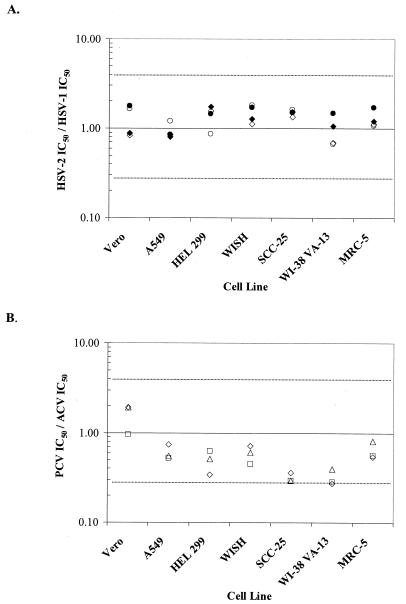
FIG. 3.
Effect of cell line on HSV type-specific susceptibility to ACV and PCV by YRA. (A) IC50 for HSV-2 333 divided by IC50 for HSV-1 SC16 (filled symbols) or IC50 for HSV-2 SB5 divided by IC50 for HSV-1 SC16 (open symbols) for both ACV (diamonds) and PCV (circles). The dashed lines represent IC50 ratios that differ by ±1 drug dilution (fourfold). (B) PCV IC50s divided by ACV IC50s for the three standard strains HSV-2 SB5 (diamonds), HSV-2 333 (triangles), and HSV-1 SC16 (squares).
Similarly, to visualize the cell-dependent differences in IC50s between ACV and PCV, the ratio of the IC50 of PCV to that of ACV was calculated (Fig. 2B). Consistent susceptibilities of both HSV types to both drugs were evident in A549, SCC-25, and Hs68 cell lines. Susceptibility to PCV was clearly lower than that to ACV in Vero cells and greater than that to ACV in WI-38 VA-13 cells. The differences in the IC50s between PCV and ACV in the remaining cell lines were less pronounced and were within ratios of 0.25 and 4.0 or 1 drug dilution in the assays used.
YRAs.
In the YRAs, multiple cycles of replication and release occur over 72 h, at the end of which progeny virus is titrated. The susceptible virus strains displayed some differential susceptibilities to PCV or ACV in the YRA, depending upon the host cell, but the impact of the host cell on the YRA was less than that seen on the PRA and did not reach significance (Table 3). Type 2 viruses were approximately twofold more susceptible to ACV than PCV when Vero cells were used, while the IC50s of PCV were essentially equivalent to those of ACV in the remaining cell lines.
TABLE 3.
IC50s for five HSV strains determined by YRA in seven cell lines
| Drug and virus | IC50 (μg/ml) in the following cell linea:
|
||||||
|---|---|---|---|---|---|---|---|
| Vero | A549 | HEL 299 | WISH | SCC-25 | WI-38 VA-13 | MRC-5 | |
| ACV | |||||||
| SB5 | 0.58 ± 0.08 | 0.62 ± 0.26 | 0.58 ± 0.37 | 0.63 ± 0.24 | 0.36 ± 0.04 | 0.51 ± 0.16 | 0.81 ± 0.01 |
| 333 | 0.62 ± 0.29 | 0.58 ± 0.22 | 0.64 ± 0.47 | 0.71 ± 0.16 | 0.41 ± 0.01 | 0.78 ± 0.15 | 0.87 ± 0.01 |
| SC16 | 0.70 ± 0.12 | 0.73 ± 0.21 | 0.37 ± 0.02 | 0.56 ± 0.4 | 0.27 ± 0.27 | 0.74 ± 0.13 | 0.73 ± 0.01 |
| SC16S1 | 23.97 ± 4.38 | 18.68 ± 8.94 | 24.11 ± 12.9 | 16.54 ± 7.04 | 13.05 ± 1.1 | 11.68 ± 0.61 | 26.41 ± 2.82 |
| DM 21 | 16.85 ± 14.14 | 18.35 ± 4.74 | 22.63 ± 8.37 | 18.81 ± 9.73 | 12.22 ± 0.74 | 20.86 ± 0.59 | 25.38 ± 8.31 |
| PCV | |||||||
| SB5 | 1.11 ± 0.64 | 0.46 ± 0.28 | 0.20 ± 0.11 | 0.45 ± 0.25 | 0.13 ± 0.05 | 0.14 ± 0.06 | 0.44 ± 0.05 |
| 333 | 1.19 ± 0.73 | 0.32 ± 0.04 | 0.33 ± 0.27 | 0.43 ± 0.28 | 0.12 ± 0.05 | 0.31 ± 0.06 | 0.70 ± 0.33 |
| SC16 | 0.67 ± 0.28 | 0.38 ± 0.13 | 0.23 ± 0.12 | 0.25 ± 0.27 | 0.08 ± 0.00 | 0.21 ± 0.08 | 0.41 ± 0.01 |
| SC16S1 | 13.57 ± 9.5 | 20.42 ± 1.17 | 9.88 ± 1.41 | 12.87 ± 1.51 | 11.81 ± 0.67 | 16.44 ± 7.46 | 18.63 ± 4.21 |
| DM 21 | 12.48 ± 6.19 | 17.44 ± 10.43 | 7.44 ± 4.67 | 16.87 ± 0.08 | 11.36 ± 0.57 | 7.86 ± 2.06 | 15.88 ± 4.49 |
Each value represents the mean ± standard deviation IC50 of three assays. The assays were done with duplicate wells over a range of five fourfold dilutions of drug.
The ratio of the IC50 for HSV-2 to the IC50 for HSV-1 determined with the IC50s from YRAs were plotted to allow a comparison of both antiviral compounds within all cell lines as well as the susceptibilities of virus types 1 and 2 (Fig. 3A). Similar susceptibilities to ACV and PCV of type 1 and type 2 viruses were evident in all cell lines, as indicated by the fact that the IC50 ratios were all in the range of 0.25 to 4.0 (Fig. 3B).
Plaque morphology.
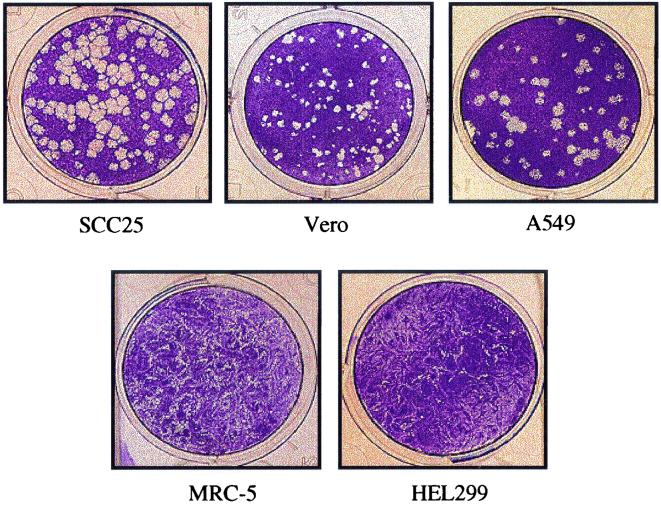
A final cell line characteristic that affects the accuracy and utility of PRA is plaque morphology. A cell line which forms easily distinguishable plaques after HSV infection greatly facilitates counting and improves the reliability of the assay. SCC-25, Vero, A549, and WI-38 VA-13 cells consistently formed well-defined plaques upon infection, regardless of the virus type or strain. The plaques generated in these cell lines were uniform in size, with an approximate mean diameter of 1.2 mm. Conversely, the fibroblastic cells, MRC-5, HEL 299, Hs68, and WISH, produced indistinct, crescent-shaped plaques which made counting technically difficult. Typical plaque morphologies on monolayers of several of the cell lines examined in this study are shown in Fig. 4.
FIG. 4.
Plaque morphology of HSV-1 SC16 in five cell lines. At 48 h postinfection, 12-well trays were fixed and stained. The plaque numbers shown here do not reflect the relative infectivity of each cell line since all plates were not infected with the same virus suspension. Additional fibroblastic cell lines, WISH, Hs68 and WI-38 VA-13 (data not shown), form plaques nearly identical to those shown for MRC-5 and HEL 299.
DISCUSSION
NCCLS has proposed a standard PRA that can be used to test the susceptibilities of HSV to ACV and FOS but not PCV (18). Both ACV and PCV are acyclic guanine derivatives with potent in vitro and in vivo activities against HSV-1 and HSV-2 (for reviews, see references 2, 6, and 29). Their antiviral activities are mediated through phosphorylation by the viral UL23 gene product, thymidine kinase (TK). The monophosphate anabolites are further phosphorylated by host enzymes, and the nucleotide triphosphates are selective inhibitors of the viral DNA polymerase, the product of the UL30 gene (10, 11, 13, 23, 32). Development of HSV resistance to both ACV and PCV occurs most often by alterations in TK, primarily through loss of enzyme activity and less commonly by alterations in the polymerase gene (5, 7, 8, 15, 27). Cross-resistance to both ACV and PCV is frequent (27). The activities of PCV in comparison with those of ACV against HSV-1, HSV-2, and varicella-zoster virus have been evaluated by several investigators in specific hosts (1, 2, 3, 9, 14, 33; for a review, see reference 4). Despite these major similarities between ACV and PCV, cell-specific differences which can affect susceptibility assay results have been reported. We undertook these experiments in order to find a cell line or lines which would minimize the known or suspected problems, maximize the desired properties, and support a standardized method for ACV and PCV susceptibility testing. We used only HSV strains with clear phenotypic pedigrees and extensive laboratory characterization in order to facilitate the interpretation of differences between cell lines, although this assumes that the results are applicable to clinical isolates. A study is under way to investigate the performance of a subset of the cell lines reported here with a panel of HSV-1 and -2 clinical isolates, and preliminary results are consistent with the conclusions reported here (data not shown).
The quantitative and qualitative criteria that we think are most important were evaluated in the cell lines and are given in Table 4, along with a summary evaluation of the cell lines tested. Two of the most important properties, susceptibility to HSV infection and ability to discriminate resistant from sensitive phenotypes, were shared by all eight cell lines. While the plating efficiency for HSV in A549 cells was threefold that in Vero cells and sixfold that in WISH cells, this is unlikely to affect their usefulness in PRA, since the differences can be overcome by varying the virus dilution. Similarly, resistant strain HSV-1 SC16S1 with altered TK (TKa) was at least 15-fold less susceptible to PCV or ACV than parental strain SC16 in all cell lines tested, according to the IC50s determined by PRA.
TABLE 4.
Cell line performance in key criteria for PRAs
| Property | Performancea of the following cell line
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Vero | A549 | HEL 299 | WISH | SCC-25 | WI-38 VA-13 | Hs68 | MRC-5 | |
| Plating efficiency | ++ | +++ | ++ | + | + | ++ | ++ | ++ |
| Resistance discrimination ≥15 times | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| HSV type independence | − | ++ | + | + | + | + | ++ | +− |
| Drug independence | − | ++ | + | − | + | − | ++ | + |
| Sensitive for IC50s <2.0 μg/ml | + | − | ++ | + | ++ | − | ++ | + |
| Robustness, longevity | +++ | +++ | − | +++ | ++ | ++ | − | − |
| Plaque morphology | +++ | ++ | + | + | ++ | ++ | + | + |
Cell line performance is summarized qualitatively as poor (−), acceptable (+), good (++), and best (+++) among the cell lines tested.
There are larger differences in other important properties. The two cell lines most frequently used for PRA (Vero and MRC-5) demonstrated the worst concordance of IC50s for type 1 strains to IC50s for type 2 strains. Since PCV IC50s for HSV-2 in these cells were up to eightfold higher than those for HSV-1, the IC50s for type 2 strains approach values often used as criteria for HSV-1 resistance. Thus, these cell lines would not appear to be a good choice for testing for susceptibility to PCV unless virus typing is an integral part of the protocol. With the remaining cell lines, the IC50s for sensitive HSV-2 concurred within 1 drug dilution, or fourfold, with the IC50 for sensitive HSV-1. Cell lines A549 and Hs68 provided the most concordant data for the two virus types.
The relative susceptibility to PCV compared to that to ACV is also dependent on the cell line. This could be important if the results of PRAs are reported as the IC50s of the two drugs. The results from tests in Vero cells might imply that an isolate is more susceptible to ACV than to PCV, while the opposite implication would hold for results from tests in WI-38 VA-13 cells. Under such circumstances, A549 cells would be a better choice since there is less bias in the IC50s between the two drugs in tests with this cell line.
An additional criterion, given the history of susceptibility testing with ACV in Vero cells, would be the ability of a cell line to produce an IC50 of less than 2.0 μg/ml for susceptible strains. Thus, the higher IC50s obtained for susceptible strains in A549 or WI-38 VA-13 cells would mean that the breakpoint would need to be revised. Fortunately, this could be solved by testing a large series of viruses in parallel with both the cell line of choice and Vero cells. The results would provide relative IC50s that could be used to normalize the breakpoints and could validate the PRA in the cell line of choice.
The final but possibly overriding criteria for choosing a cell line are the ease of use, including plaque visibility, technical robustness, and long-term stability of the host cell line. From this perspective, all of the diploid, fibroblastic cells would be less advantageous. These cells can be more difficult to culture, generally take longer to grow, and have limited life spans. Stocks of MRC-5 cells are now at passage levels that severely limit the number of additional passages possible, since higher passages may affect HSV infection (19). Furthermore, HSV plaques in these cell types, exemplified by MRC-5 cells, are poorly differentiated, and more skill and attention are required to count plaques in these cells than in the more epithelial cell lines like Vero and A549 cells.
Among the eight cells lines tested, no single cell line is ideal for all PCV and ACV PRAs. As seen in Table 4, A549 cells meet most of the important criteria, but validation of breakpoint IC50s is needed. Vero, MRC-5, and HEL 299 cells also have many desirable properties which make them highly useful in specific circumstances, but they provide data which may need more complicated assessment or interpretation, and the last two lines provide poor plaque morphologies.
It is interesting that the impacts of the host cell line on the parameters measured were more pronounced by PRA than by YRA. Antiviral agents are often ranked according to their performance in a PRA, although it may be of greater relevance to test the ability of a compound to inhibit the production of infectious virus, as in the YRA, rather than the formation of a plaque. YRA can be a more sensitive test than PRA and reflects the ability of a compound to both affect virus replication and spread throughout the culture. It is possible that specific events that are important for plaque formation, such as spread by cell-to-cell contact and cell lysis, also vary with cell type and affect perceived drug inhibition rather than actual virus replication. This argues for yield-type assays which measure a virus end product, such as a protein enzyme-linked immunosorbent assay or a genomic DNA assay (24, 31). Such assays are also easier to use than PRA, especially as commercial kits.
The antiviral activity of FOS does not require HSV TK expression or cellular phosphorylation. This analogue was included to test whether phosphorylation mechanisms might explain cell-to-cell differences in antiviral potency. Interestingly, the dichotomy between the ACV and PCV IC50s for HSV-2 and HSV-1 seen in Vero and WI-38 VA-13 cells was not seen with FOS. This suggests the hypothesis that this wide variation in IC50 ratios is dependent upon the relative activities of host kinases in the two cell lines. However, it is also noteworthy that variations in FOS IC50s from 20 to over 100 μg/ml were observed for susceptible HSV strains in cell lines other than Vero and HEL 299 cells. Thus, a need for FOS susceptibility testing in parallel with ACV and PCV susceptibility testing should be an additional criterion to be considered when choosing a cell line or lines for a particular analysis. In addition, since the activity of FOS is not host phosphorylation dependent, kinase activity variations cannot account for all of the differences in the antiviral activities by cell line reported here.
Acknowledgments
We thank Bob Gagnon for statistical analysis; Clyde Crumpacker, Graham Darby, and Richard Hodinka for helpful discussions; Priscilla Schaffer for the gift of HSV-2 333; and Sharon Safrin for the gift of HSV-1 SC16, SC16S1, and DM21. We also appreciate the support and critical review of Klaus Esser.
The financial support of Ron Boon and Teresa Bacon of SmithKline Beecham Consumer Healthcare is gratefully acknowledged.
REFERENCES
- 1.Bacon, T. H., B. A. Howard, L. C. Spender, and M. R. Boyd. 1996. Activity of penciclovir in antiviral assays against herpes- simplex virus. J. Antimicrob. Chemother. 37:303-313. [DOI] [PubMed] [Google Scholar]
- 2.Bacon, T. H., and R. F. Schinazi. 1993. An overview of the further evaluation of penciclovir against herpes simplex virus and varicella-zoster virus in cell culture highlighting contrasts with acyclovir. Antivir. Chem. Chemother. 4:25-36. [Google Scholar]
- 3.Boyd, M. R., T. H. Bacon, D. Sutton, and M. Cole. 1987. Antiherpesvirus activity of 9-(4-hydroxy-3-hydroxy-methylbut-1-yl)guanine (BRL 39123) in cell culture. Antimicrob. Agents Chemother. 31:1238-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd, M. R., S. Safrin, and E. R. Kern. 1993. Penciclovir: a review of its spectrum of activity selectivity and cross-resistance pattern. Antivir. Chem. Chemother. 4:3-11. [Google Scholar]
- 5.Coen, D. M., and P. A. Schaffer. 1980. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc. Natl. Acad. Sci. USA 77:2265-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, P. 1983. The spectrum of antiviral activities of acyclovir in vitro and in vivo. J. Antimicrob. Chemother. 12(Suppl. B):19-27. [DOI] [PubMed] [Google Scholar]
- 7.Crumpacker, C. S., L. E. Schnipper, S. I. Marlowe, P. N. Kowalsky, B. J. Hershey, and M. J. Levin. 1982. Resistance to antiviral drugs of herpes simplex virus isolated from a patient treated with acyclovir. N. Engl. J. Med. 306:343-346. [DOI] [PubMed] [Google Scholar]
- 8.Darby, G., H. J. Field, and S. A. Salisbury. 1981. Altered substrate specificity of herpes simplex virus thymidine kinase confers acyclovir-resistance. Nature 289:81-83. [DOI] [PubMed] [Google Scholar]
- 9.Datema, R., A. C. Ericson, H. J. Field, A. Larsson, and K. Stenberg. 1987. Critical determinants of antiherpes efficacy of buciclovir and related acyclic guanosine analogs. Antivir. Res. 7:303-316. [DOI] [PubMed] [Google Scholar]
- 10.Derse, D., Y. C. Cheng, P. A. Furman, M. H. St. Clair, and G. B. Elion. 1981. Inhibition of purified human and herpes simplex virus-induced DNA polymerases by 9-(2-hydroxyethoxymethyl)guanine triphosphate. Effects on primer-template function. J. Biol. Chem. 256:11447-11451. [PubMed] [Google Scholar]
- 11.Earnshaw, D. L., T. H. Bacon, S. J. Darlison, K. Edmonds, R. M. Perkins, and R. A. VereHodge. 1992. Mode of antiviral action of penciclovir in MRC-5 cells infected with herpes simplex virus type 1 (HSV-1), HSV-2, and varicella-zoster virus. Antimicrob. Agents Chemother. 36:2747-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Efstathiou, S., S. Kemp, G. Darby, and A. C. Minson. 1989. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J. Gen. Virol. 70:869-879. [DOI] [PubMed] [Google Scholar]
- 13.Elion, G. B. 1993. Acyclovir: discovery, mechanism of action, and selectivity. J. Med. Virol. Suppl. 1:2-6. [DOI] [PubMed]
- 14.Ertl, P., W. Snowden, D. Lowe, W. Miller, P. Collins, and E. Littler. 1995. A comparative study of the in vitro and invivo antiviral activities of acyclovir and penciclovir. Antivir. Chem. Chemother. 6:89-97. [Google Scholar]
- 15.Field, H. J., B. A. Larder, and G. Darby. 1982. Isolation and characterization of acyclovir-resistant strains of herpes simplex virus. Am. J. Med. 73:369-371. [DOI] [PubMed] [Google Scholar]
- 16.Harmenberg, J., V. A. Sundqvist, H. Gadler, B. Leven, G. Brannstrom, and B. Wahren. 1986. Comparative methods for detection of thymidine kinase-deficient herpes simplex virus type 1 strains. Antimicrob. Agents Chemother. 30:570-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harmenberg, J., B. Wahren, and B. Oberg. 1980. Influence of cells and virus multiplicity on the inhibition of herpesviruses with acycloguanosine. Intervirology 14:239-244. [DOI] [PubMed] [Google Scholar]
- 18.Hodinka, R., E. Swierkosz, D. Lancaster, B. M. Moore, S. Sacks, D. Scholl, and D. K. Wright. 2000. Antiviral susceptibility testing. Proposed standard M33-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.Jensen, H. L., and B. Norrild. 2000. The effects of cell passages on the cell morphology and the outcome of herpes simplex virus type 1 infection. J. Virol. Methods 84:139-152. [DOI] [PubMed] [Google Scholar]
- 20.Kärber, G. 1931. Bertrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmakol. 162:480-483. [Google Scholar]
- 21.Larsson, A., K. Stenberg, A. C. Ericson, U. Haglund, W. A. Yisak, N. G. Johansson, B. Oberg, and R. Datema. 1986. Mode of action, toxicity, pharmacokinetics, and efficacy of some new antiherpesvirus guanosine analogs related to buciclovir. Antimicrob. Agents Chemother. 30:598-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaren, C., M. N. Ellis, and G. A. Hunter. 1983. A colorimetric assay for the measurement of the sensitivity of herpes simplex viruses to antiviral agents. Antivir. Res. 3:223-234. [DOI] [PubMed] [Google Scholar]
- 23.Miller, W. H., and R. L. Miller. 1982. Phosphorylation of acyclovir diphosphate by cellular enzymes. Biochem. Pharmacol. 31:3879-3884. [DOI] [PubMed] [Google Scholar]
- 24.Rabalais, G. P., M. J. Levin, and F. E. Berkowitz. 1987. Rapid herpes simplex virus susceptibility testing using an enzyme-linked immunosorbent assay performed in situ on fixed virus-infected monolayers. Antimicrob. Agents Chemother. 31:946-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Safrin, S., T. Elbeik, L. Phan, D. Robinson, J. Rush, A. Elbaggari, and J. Mills. 1994. Correlation between response to acyclovir and foscarnet therapy and in vitro susceptibility result for isolates of herpes simplex virus from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 38:1246-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safrin, S., L. Phan, and T. Elbeik. 1995. A comparative evaluation of three methods of antiviral susceptibility testing of clinical herpes simplex virus isolates. Clin. Diagn. Virol. 4:81-91. [DOI] [PubMed] [Google Scholar]
- 27.Sarisky, R. T., M. R. Quail, P. E. Clark, T. T. Nguyen, W. S. Halsey, R. J. Wittrock, J. O. Bartus, M. M. Van Horn, G. M. Sathe, S. Van Horn, M. D. Kelly, T. H. Bacon, and J. J. Leary. 2001. Characterization of herpes simplex viruses selected in culture for resistance to penciclovir or acyclovir. J. Virol. 75:1761-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Standring-Cox, R., T. H. Bacon, and B. A. Howard. 1996. Comparison of a DNA probe assay with the plaque reduction assay for measuring the sensitivity of herpes simplex virus and varicella-zoster virus to penciclovir and acyclovir. J. Virol. Methods 56:3-11. [DOI] [PubMed] [Google Scholar]
- 29.Sutton, D., and E. R. Kern. 1993. Activity of famciclovir and penciclovir in HSV-infected animals: a review. Antivir. Chem. Chemother. 4:37-46. [Google Scholar]
- 30.Swierkosz, E. M., and K. K. Biron. 1995. Antiviral agents and susceptibility testing, p. 1415-1423. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. ASM Press, Washington, D. C.
- 31.Swierkosz, E. M., D. R. Scholl, J. L. Brown, J. D. Jollick, and C. A. Gleaves. 1987. Improved DNA hybridization method for detection of acyclovir-resistant herpes simplex virus. Antimicrob. Agents Chemother. 31:1465-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VereHodge, R. A., and R. M. Perkins. 1989. Mode of action of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) against herpes simplex virus in MRC-5 cells. Antimicrob. Agents Chemother. 33:223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberg, A., B. J. Bate, H. B. Masters, S. A. Schneider, J. C. Clark, C. G. Wren, J. A. Allaman, and M. J. Levin. 1992. In vitro activities of penciclovir and acyclovir against herpes simplex virus types 1 and 2. Antimicrob. Agents Chemother. 36:2037-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]