Abstract
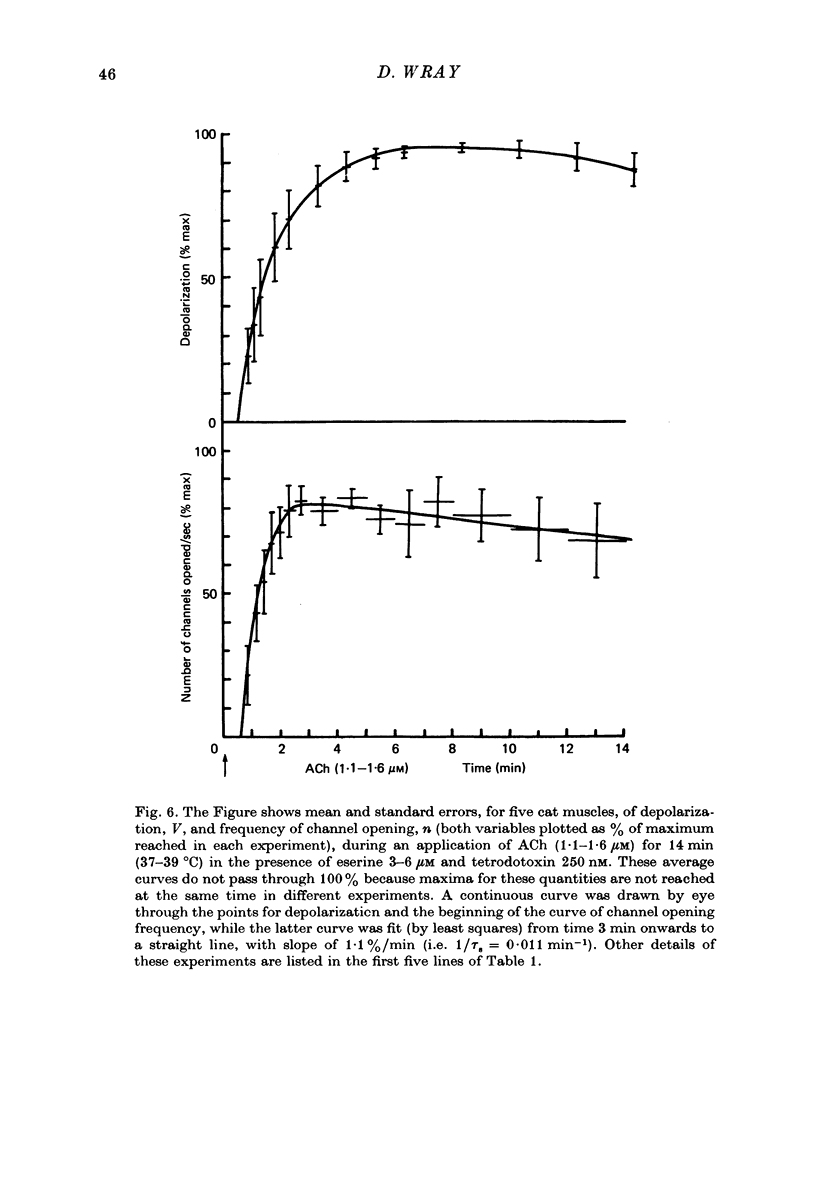
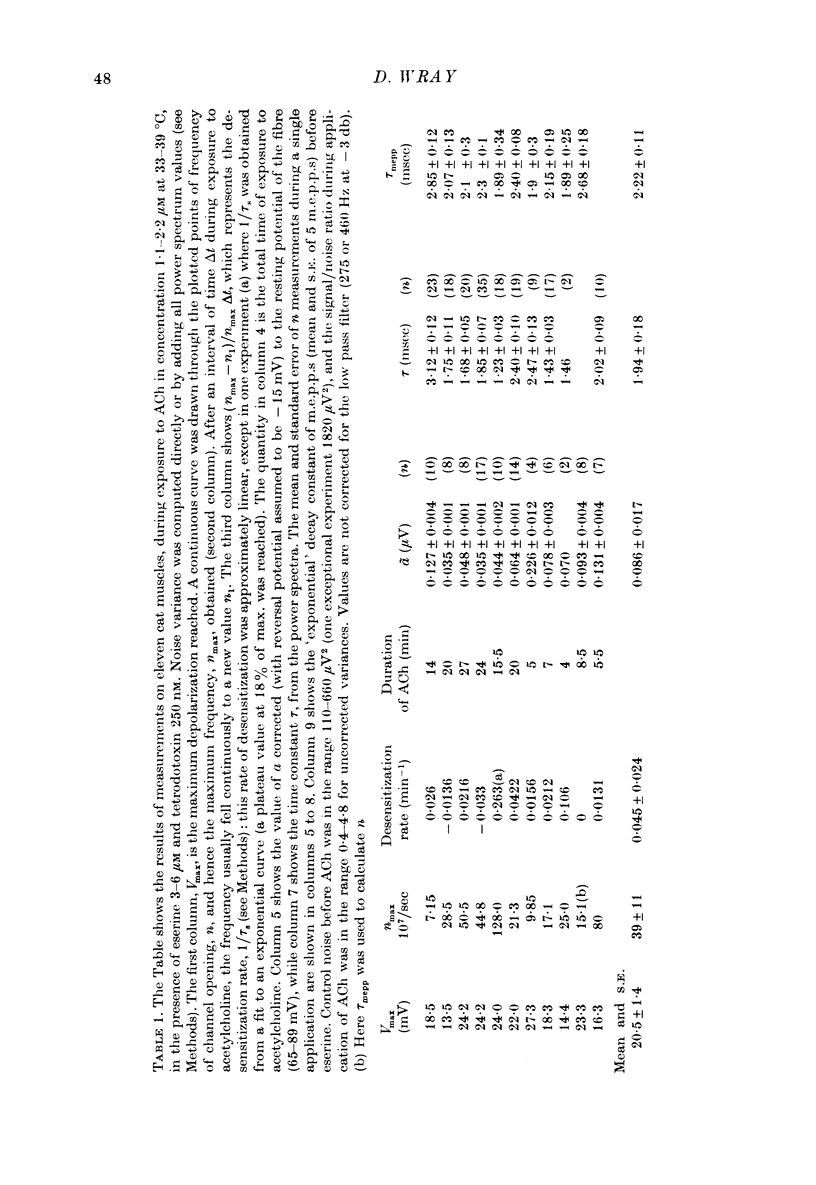
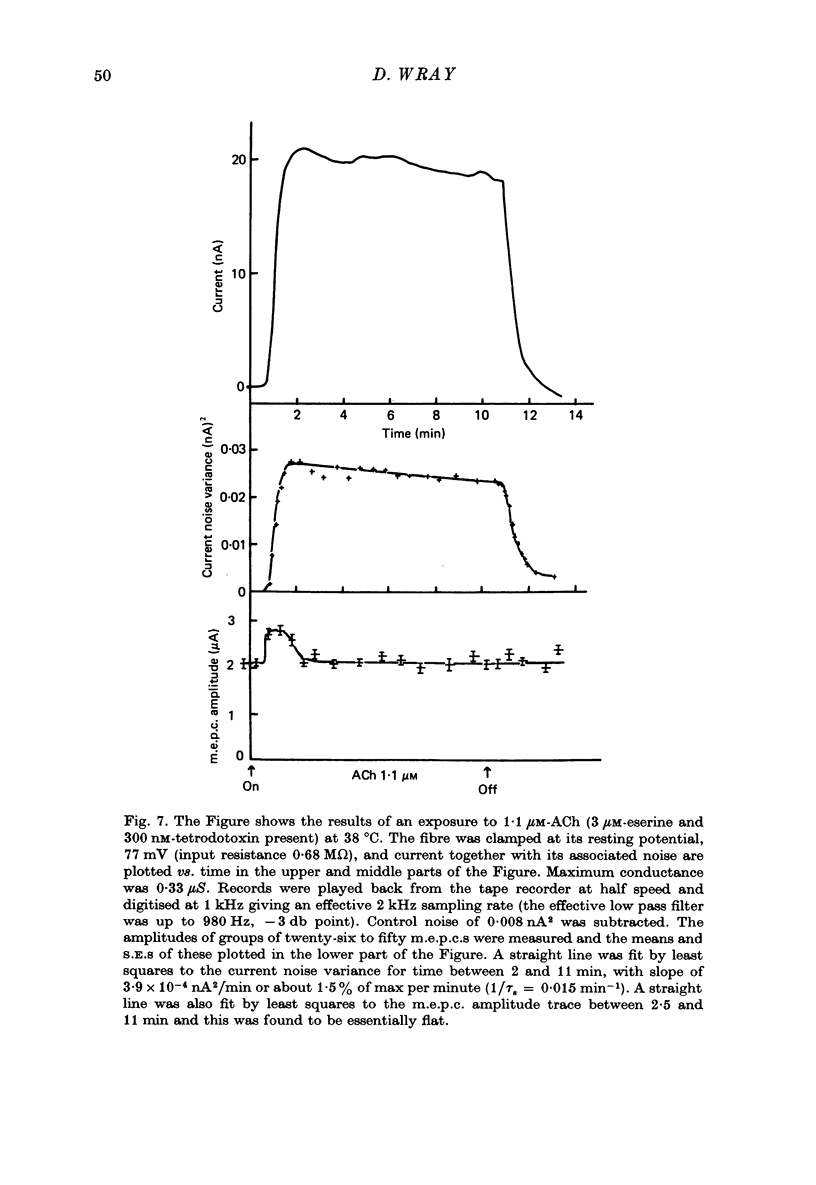
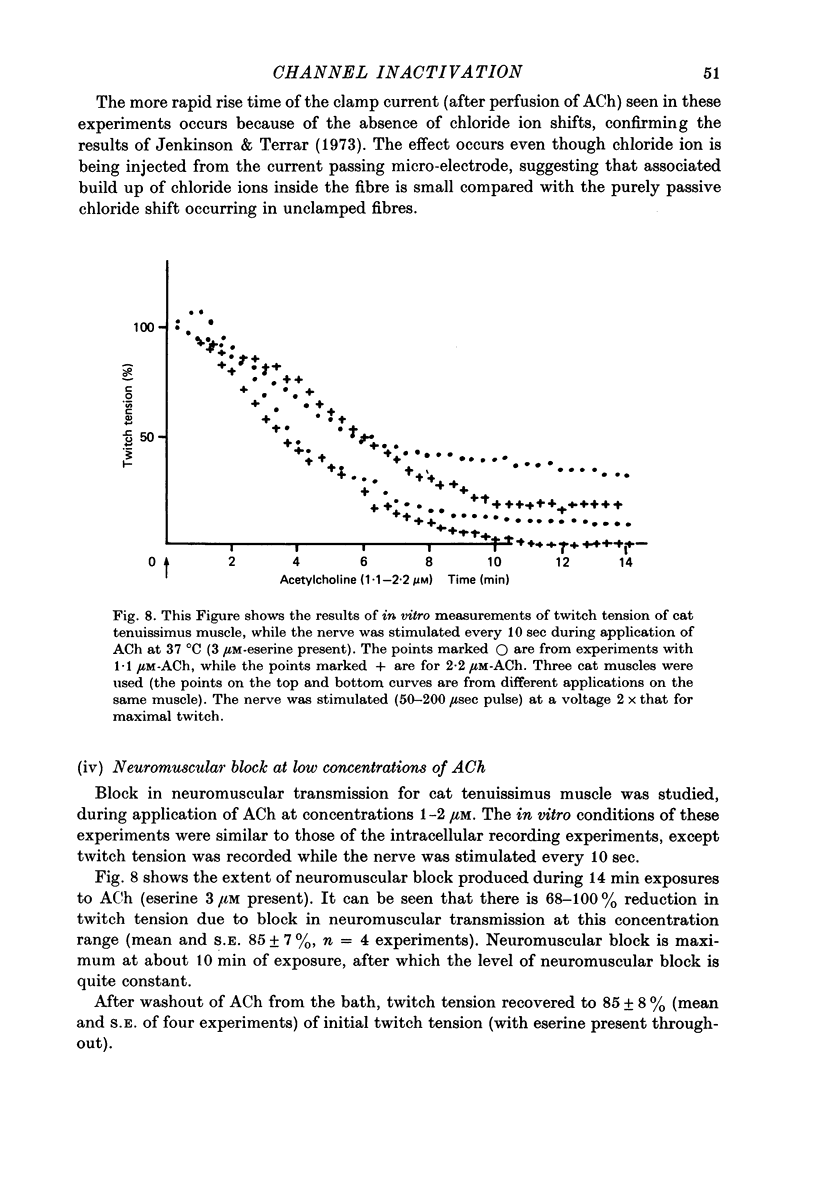
1. Micro-electrodes were used to record membrane potential and associated noise at the end-plate region of cat tenuissimus muscle (37 degrees C), during applications of acetylcholine (ACh) in continuously flowing Krebs solution containing eserine and tetrodotoxin. 2. Densensitization was assessed from the frequency of channel opening calculated from the noise variance. 3. At higher concentrations of ACh (10-50 microM), desensitization occurred with an exponential fall to a plateau. 4. At low concentrations of ACh (1-2 microM) only slight desensitization occurred and at a much lower rate. Frequency of channel opening decreased at the rate of 0.045 +/- 0.024 min-1. Maximum frequency was (33 +/- 9) X 10(7)/sec while maximum depolarization was 20.5 +/- 1.4 mV (n = 11 cats). Depolarization was well maintained. 5. This slow rate of desensitization at low concentrations of ACh was confirmed in experiments where voltage clamped current, its associated noise, and miniature end-plate current amplitude were measured. 6. At low concentrations of ACh (1-2 microM) in the presence of eserine there was sustained block in neuromuscular transmission when twitch tension was measured. 8. It is concluded that the mechanism of neuromuscular block by ACh at around 1 microM concentration is by depolarization itself, not desensitization.
Full text
PDF




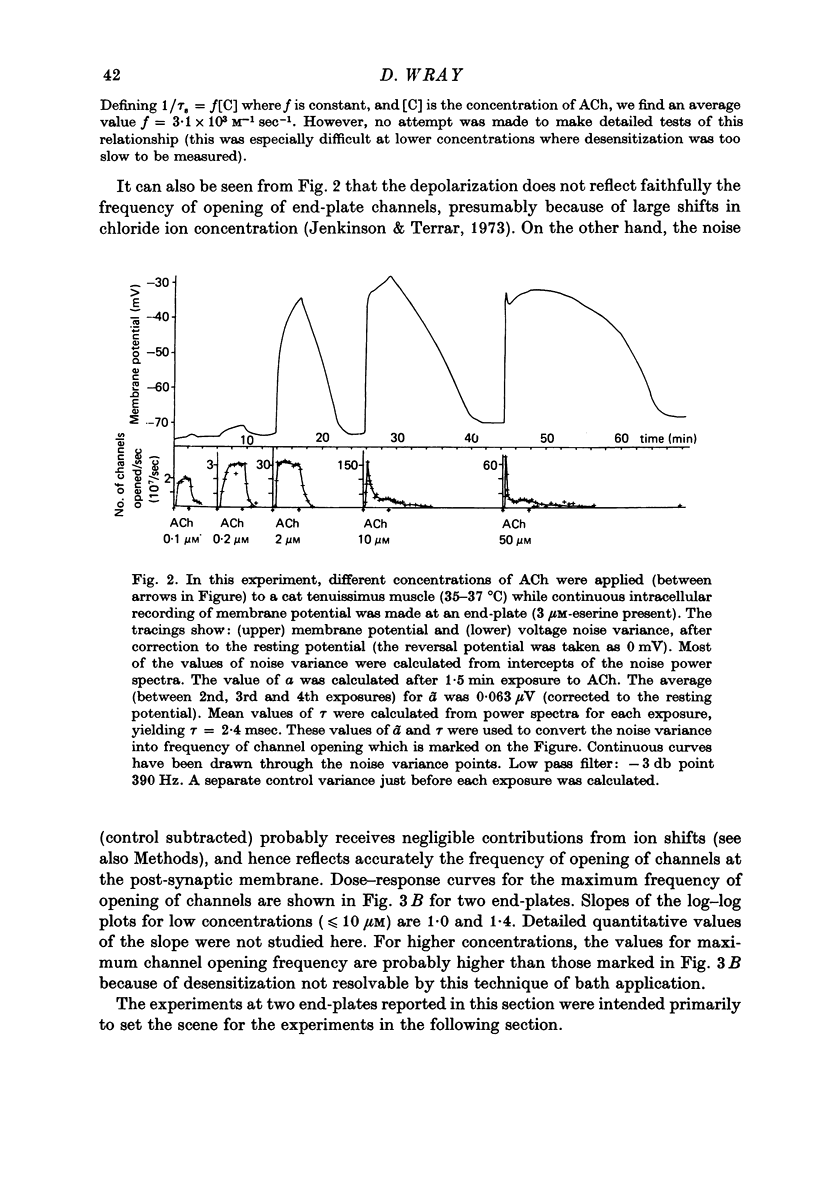
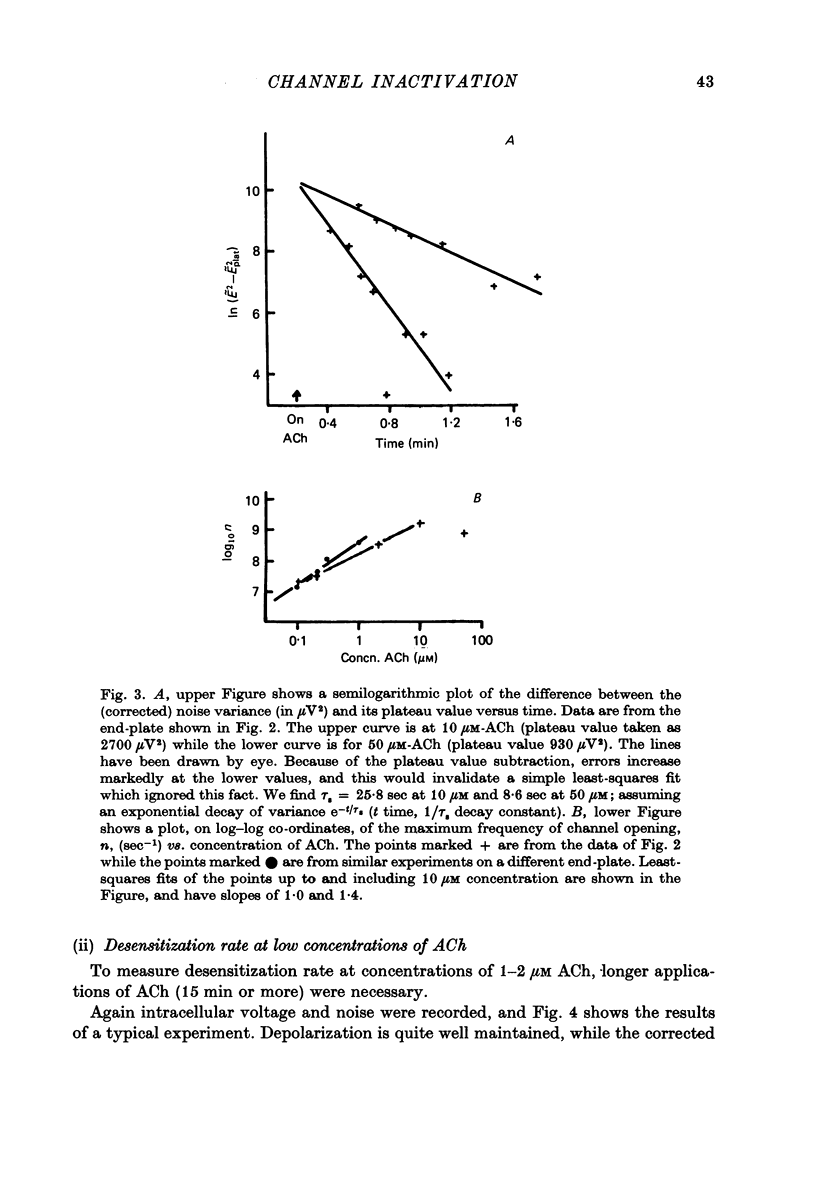
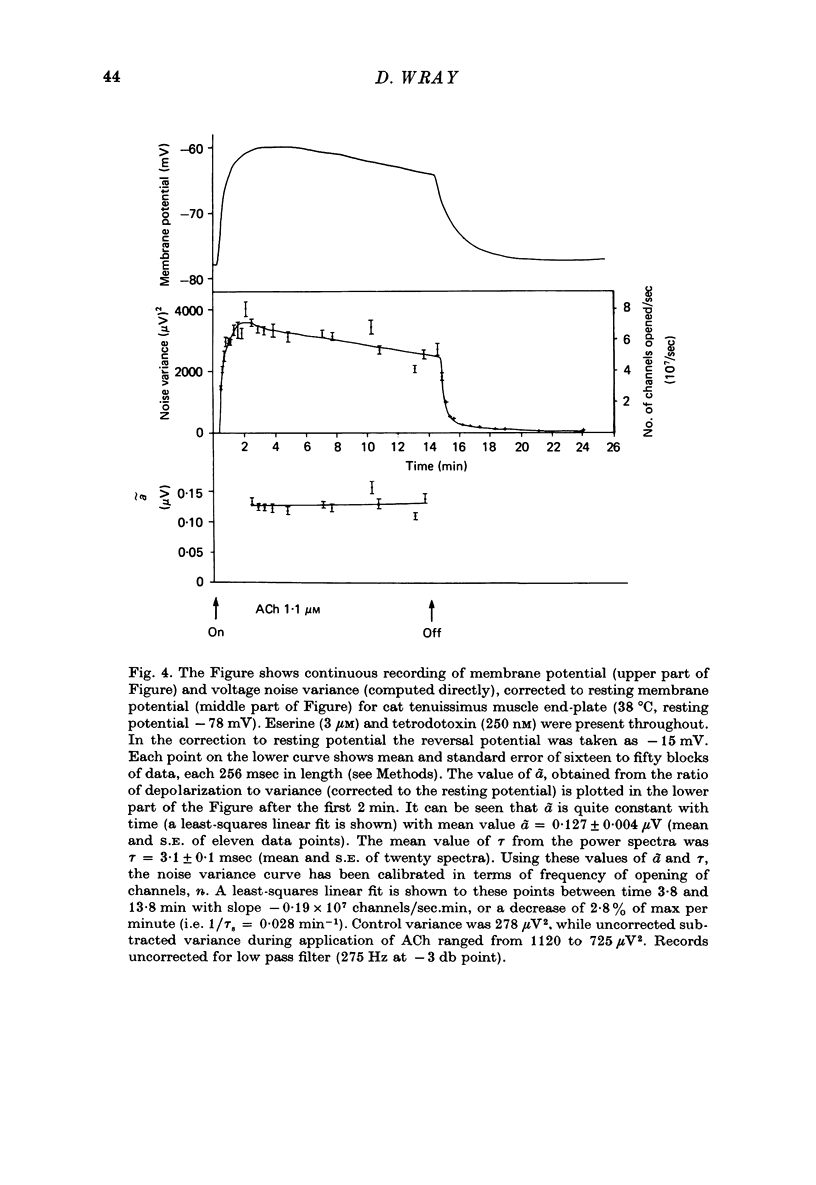
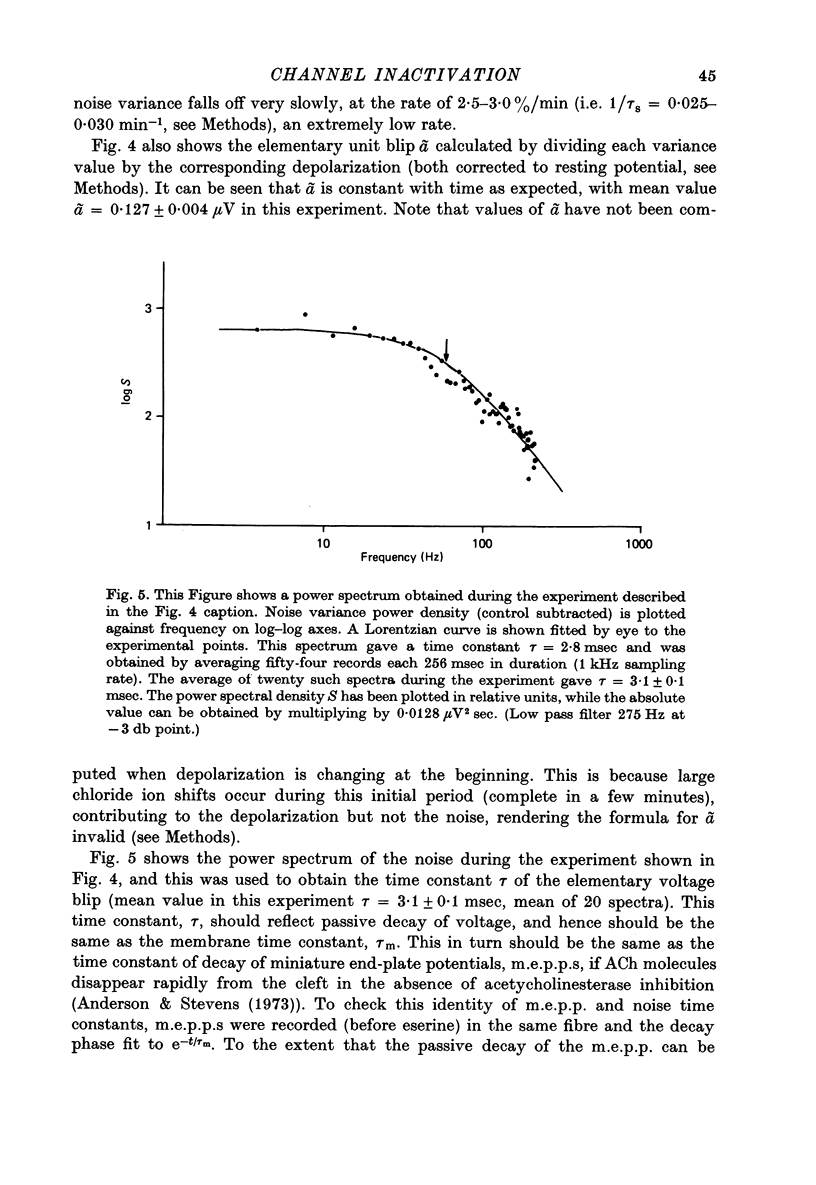







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELSSON J., THESLEFF S. The desensitizing effect of acetylcholine on the mammalian motor end-plate. Acta Physiol Scand. 1958 Jul 17;43(1):15–26. doi: 10.1111/j.1748-1716.1958.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Adams P. R. A study of desensitization using voltage clamp. Pflugers Arch. 1975 Oct 28;360(2):135–144. doi: 10.1007/BF00580536. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. Membrane constants of mammalian muscle fibres. J Physiol. 1959 Oct;147:450–457. doi: 10.1113/jphysiol.1959.sp006255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. Spontaneous subthreshold activity at mammalian neural muscular junctions. J Physiol. 1956 Apr 27;132(1):61–73. doi: 10.1113/jphysiol.1956.sp005502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNS B. D., PATON W. D. M. Depolarization of the motor end-plate by decamethonium and acetylcholine. J Physiol. 1951 Sep;115(1):41–73. doi: 10.1113/jphysiol.1951.sp004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacq Z. M., Brown G. L. Pharmacological experiments on mammalian voluntary muscle, in relation to the theory of chemical transmission. J Physiol. 1937 Feb 19;89(1):45–60. doi: 10.1113/jphysiol.1937.sp003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Large W. A., Rang H. P. An analysis of the action of a false transmitter at the neuromuscular junction. J Physiol. 1977 Apr;266(2):361–395. doi: 10.1113/jphysiol.1977.sp011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D. Mechanisms of drug action at the voluntary muscle endplate. Annu Rev Pharmacol. 1975;15:307–325. doi: 10.1146/annurev.pa.15.040175.001515. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The membrane change produced by the neuromuscular transmitter. J Physiol. 1954 Sep 28;125(3):546–565. doi: 10.1113/jphysiol.1954.sp005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore D. I., Nastuk W. L. Ionophore-mediated calcium influx effects on the post-synaptic muscle fibre membrane. Nature. 1977 Dec 1;270(5636):441–443. doi: 10.1038/270441a0. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P. The electromotive action of acetylcholine at the motor end-plate. J Physiol. 1950 Oct 16;111(3-4):408–422. doi: 10.1113/jphysiol.1950.sp004492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENERICK H. P., GERARD R. W. Membrane potential and threshold of single muscle fibers. J Cell Physiol. 1953 Aug;42(1):79–102. doi: 10.1002/jcp.1030420106. [DOI] [PubMed] [Google Scholar]
- JENKINSON D. H. The antagonism between tubocurarine and substances which depolarize the motor end-plate. J Physiol. 1960 Jul;152:309–324. doi: 10.1113/jphysiol.1960.sp006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson D. H., Terrar D. A. Influence of chloride ions on changes in membrane potential during prolonged application of carbachol to frog skeletal muscle. Br J Pharmacol. 1973 Feb;47(2):363–376. doi: 10.1111/j.1476-5381.1973.tb08334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of procaine on the action of acetylcholine at the neuromuscular junction. J Physiol. 1975 Jul;249(2):269–284. doi: 10.1113/jphysiol.1975.sp011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The reversal potential at the desensitized endplate. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):329–334. doi: 10.1098/rspb.1977.0144. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. H., Spannbauer P. M., Parsons R. L. Desensitisation does not selectively alter sodium channels. Nature. 1977 Aug 11;268(5620):553–555. doi: 10.1038/268553a0. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazanik L. G., Vyskocil F. Dependence of acetylcholine desensitization on the membrane potential of frog muscle fibre and on the ionic changes in the medium. J Physiol. 1970 Oct;210(3):507–518. doi: 10.1113/jphysiol.1970.sp009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W. D. M., ZAIMIS E. The methonium. Pharmacol Rev. 1952 Sep;4(3):219–253. [PubMed] [Google Scholar]
- Rang H. P. Acetylcholine receptors. Q Rev Biophys. 1974 Jul;7(3):283–399. doi: 10.1017/s0033583500001463. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Ritter J. M. On the mechanism of desensitization at cholinergic receptors. Mol Pharmacol. 1970 Jul;6(4):357–382. [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray D. Frequency of opening of channels by depolarizing drugs [proceedings]. J Physiol. 1978 Nov;284:149P–150P. [PubMed] [Google Scholar]
- ZAIMIS E. Factors influencing the action of neuromuscular blocking substances. Lect Sci Basis Med. 1956;VOL:208–216. [PubMed] [Google Scholar]


