Abstract
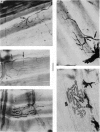
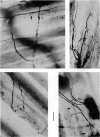
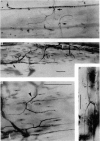
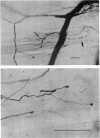
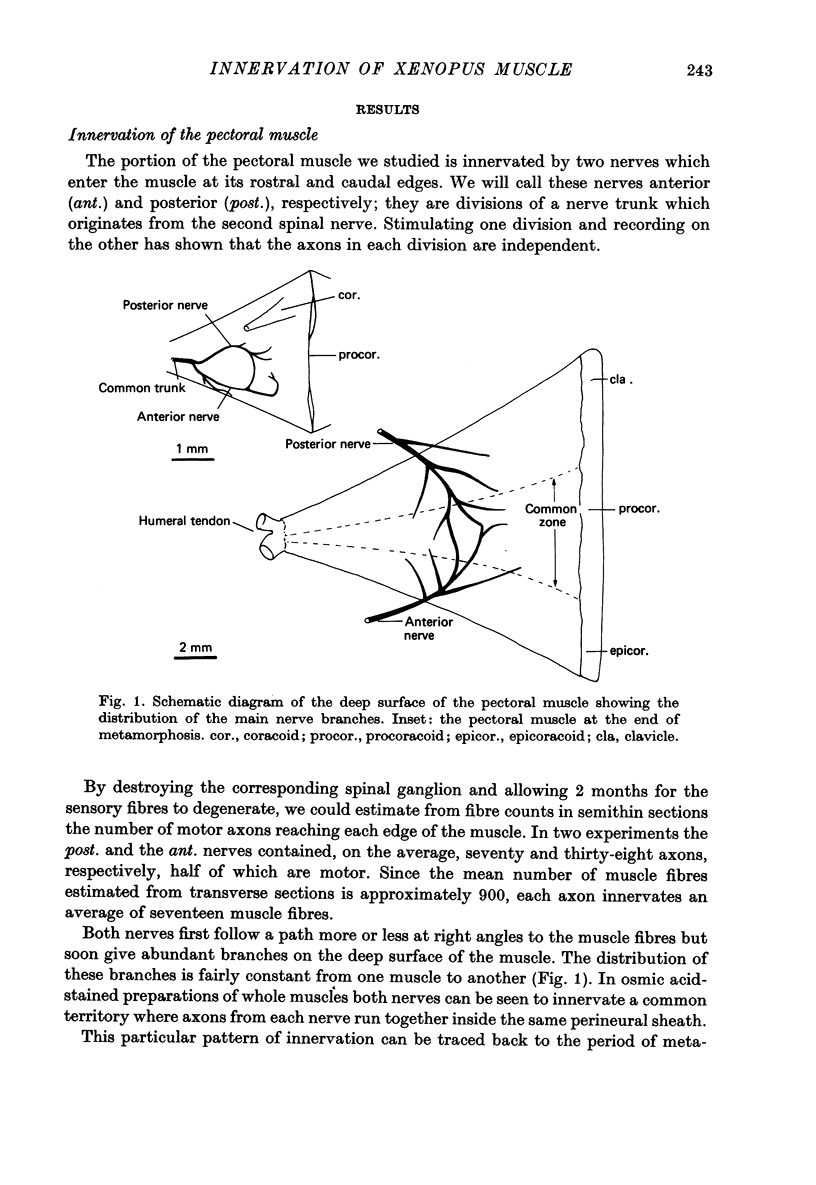
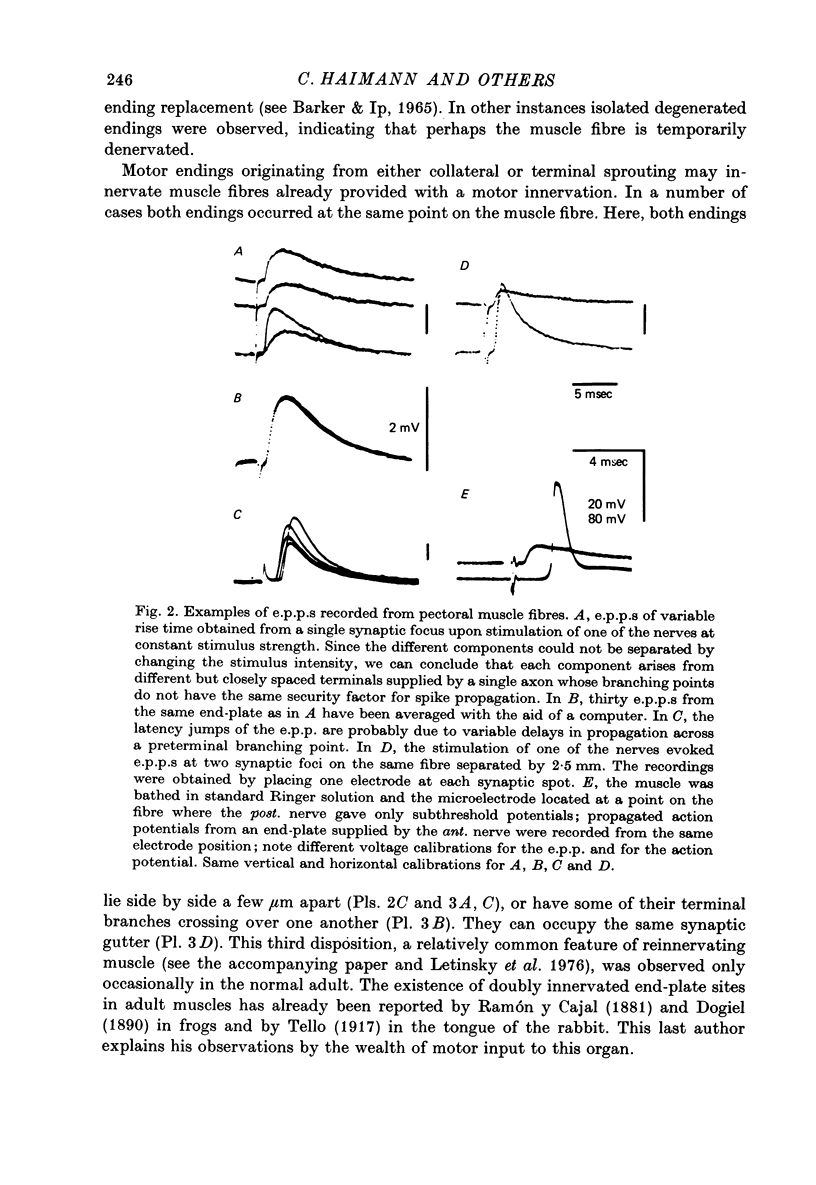
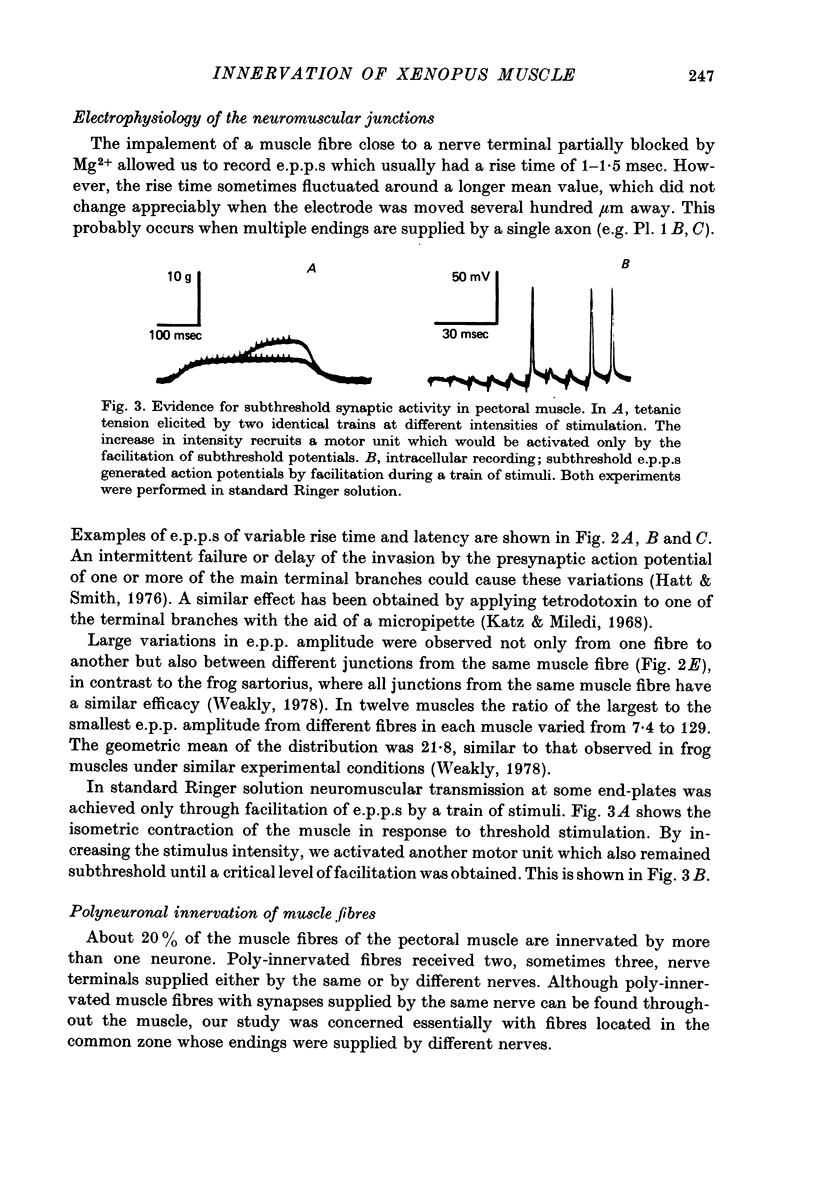
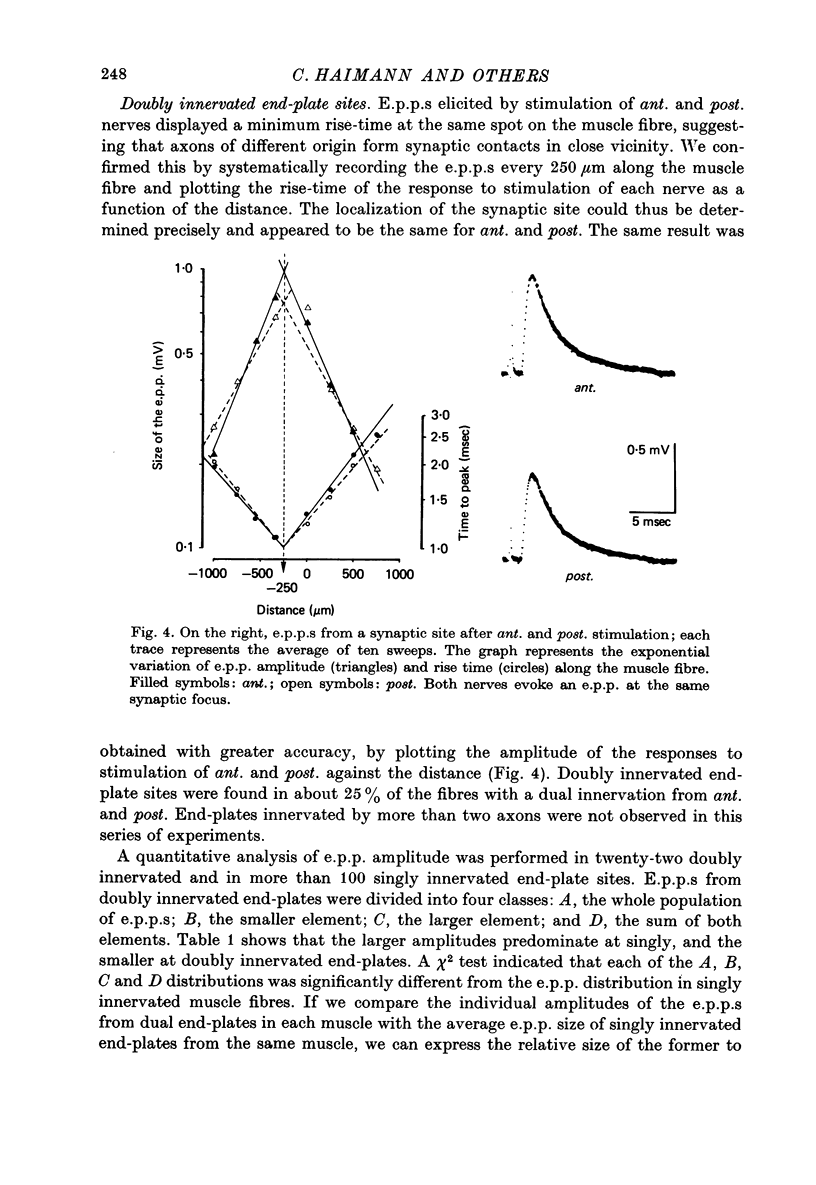
1. An anatomical and electrophysiological study was performed on the pectoral muscle of Xenopus laevis. 2. Silver-impregnated preparations revealed immature endings, collateral and terminal sprouting and signs of synaptic regression. 3. Twenty percent of the fibres received a dual innervation from two different nerves. The synapses of 25% of these fibres are formed in close vicinity. 4. Some of the singly innervated and most of the dually innervated end-plates generated only subthreshold electrical activity. Synaptic efficacy in dually innervated muscle fibres with closely spaced or distant endings was, on the average, one third and two thirds, respectively, of that obtained in singly innervated fibres. 5. Fibres with subthreshold electrical activity displayed normal ACh sensitivity. 6. The existence of non-transmitting synapses, of dually innervated end-plate sites and of morphological signs of the sprouting of new endings and the degeneration of old ones suggests that synaptic remodelling may occur in normal adult muscles.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angaut-Petit D., Mallart A. Dual innervation of end-plate sites and its consequences for neuromuscular transmission in muscles of adult Xenopus laevis. J Physiol. 1979 Apr;289:203–218. doi: 10.1113/jphysiol.1979.sp012733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN M. C., MATTHEWS P. B. An investigation into the possible existence of polyneuronal innervation of individual skeletal muscle fibres in certain hind-limb muscles of the cat. J Physiol. 1960 Jun;151:436–457. doi: 10.1113/jphysiol.1960.sp006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D., Ip M. C. Sprouting and degeneration of mammalian motor axons in normal and de-afferentated skeletal muscle. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):538–554. doi: 10.1098/rspb.1966.0008. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in amphibian striated muscle during development. J Physiol. 1975 Oct;252(1):203–239. doi: 10.1113/jphysiol.1975.sp011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Raftos J. The formation and regression of synapses during the re-innervation of axolotl striated muscles. J Physiol. 1977 Feb;265(2):261–295. doi: 10.1113/jphysiol.1977.sp011716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray J. J., Harris A. J. Dissociation between nerve-muscle transmission and nerve trophic effects on rat diaphragm using type D botulinum toxin. J Physiol. 1975 Dec;253(1):53–77. doi: 10.1113/jphysiol.1975.sp011179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Characteristics of transmitter release at regenerating frog neuromuscular junctions. J Physiol. 1974 Jun;239(3):571–594. doi: 10.1113/jphysiol.1974.sp010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Yip J. W. Formation and elimination of foreign synapses on adult salamander muscle. J Physiol. 1978 Jan;274:299–310. doi: 10.1113/jphysiol.1978.sp012148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J., Cooper E., Turner C., Macintyre L. Trophic regulation of nerve sprouting. Science. 1976 Jul 30;193(4251):371–377. doi: 10.1126/science.935873. [DOI] [PubMed] [Google Scholar]
- Frank E., Jansen J. K. Interaction between foreign and original nerves innervating gill muscles in fish. J Neurophysiol. 1976 Jan;39(1):84–90. doi: 10.1152/jn.1976.39.1.84. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Letinsky M. S., Rheuben M. B. Competitive interaction between foreign nerves innervating frog skeletal muscle. J Physiol. 1979 Apr;289:241–262. doi: 10.1113/jphysiol.1979.sp012735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatt H., Smith D. O. Synaptic depression related to presynaptic axon conduction block. J Physiol. 1976 Jul;259(2):367–393. doi: 10.1113/jphysiol.1976.sp011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip M. C. Some morphological features of the myoneural junctions in certain normal muscles of the rat. Anat Rec. 1974 Dec;180(4):605–615. doi: 10.1002/ar.1091800407. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J. THE LOCALIZATION OF CHOLINESTERASE ACTIVITY IN RAT CARDIAC MUSCLE BY ELECTRON MICROSCOPY. J Cell Biol. 1964 Nov;23:217–232. doi: 10.1083/jcb.23.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of local blockage of motor nerve terminals. J Physiol. 1968 Dec;199(3):729–741. doi: 10.1113/jphysiol.1968.sp008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler D., Thompson W., Jansen J. K. The elimination of synapses in multiply-innervated skeletal muscle fibres of the rat: dependence on distance between end-plates. Brain Res. 1977 Dec 16;138(2):353–358. doi: 10.1016/0006-8993(77)90752-1. [DOI] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinsky M. S. Acetylcholine sensitivity changes in tadpole tail muscle fibers innervated by developing motor neurons. J Neurobiol. 1975 Nov;6(6):609–617. doi: 10.1002/neu.480060607. [DOI] [PubMed] [Google Scholar]
- Letinsky M. S., Fischbeck K. H., McMahan U. J. Precision of reinnervation of original postsynaptic sites in frog muscle after a nerve crush. J Neurocytol. 1976 Dec;5(6):691–718. doi: 10.1007/BF01181582. [DOI] [PubMed] [Google Scholar]
- Letinsky M. S. The development of nerve-muscle junctions in Rana catesbeiana tadpoles. Dev Biol. 1974 Sep;40(1):129–153. doi: 10.1016/0012-1606(74)90114-6. [DOI] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H. Control of ACh sensitivity in rat muscle fibers. Cold Spring Harb Symp Quant Biol. 1976;40:263–274. doi: 10.1101/sqb.1976.040.01.027. [DOI] [PubMed] [Google Scholar]
- Magherini P. C., Precht W. Electrical properties of frog motoneurons in the in situ spinal cord. J Neurophysiol. 1976 May;39(3):459–473. doi: 10.1152/jn.1976.39.3.459. [DOI] [PubMed] [Google Scholar]
- Nasledov G. A., Thesleff S. Denervation changes in frog skeletal muscle. Acta Physiol Scand. 1974 Feb;90(2):370–380. doi: 10.1111/j.1748-1716.1974.tb05598.x. [DOI] [PubMed] [Google Scholar]
- O'Brien R. A., Ostberg A. J., Vrbová G. Observations on the elimination of polyneuronal innervation in developing mammalian skeletal muscle. J Physiol. 1978 Sep;282:571–582. doi: 10.1113/jphysiol.1978.sp012482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORKAND R. K. A further study of electrical responses in slow and twitch muscle fibres of the frog. J Physiol. 1963 Jun;167:181–191. doi: 10.1113/jphysiol.1963.sp007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley D. A. Spontaneous elimination of nerve terminals from the endplates of developing skeletal myofibers. Brain Res. 1977 Oct 7;134(2):279–285. doi: 10.1016/0006-8993(77)91073-3. [DOI] [PubMed] [Google Scholar]
- Rotshenker S., McMahan U. J. Altered patterns of innervation in frog muscle after denervation. J Neurocytol. 1976 Dec;5(6):719–730. doi: 10.1007/BF01181583. [DOI] [PubMed] [Google Scholar]
- Smith R. S., Lännergren J. Types of motor units in the skeletal muscle of Xenopus laevis. Nature. 1968 Jan 20;217(5125):281–283. doi: 10.1038/217281a0. [DOI] [PubMed] [Google Scholar]
- Smith R. S., Ovalle W. K., Jr Varieties of fast and slow extrafusal muscle fibres in amphibian hind limb muscles. J Anat. 1973 Oct;116(Pt 1):1–24. [PMC free article] [PubMed] [Google Scholar]
- Sotelo C., Palay S. L. Altered axons and axon terminals in the lateral vestibular nucleus of the rat. Possible example of axonal remodeling. Lab Invest. 1971 Dec;25(6):653–671. [PubMed] [Google Scholar]
- Tonge D. A. Effect of implantation of an extra nerve on the recovery of neuromuscular transmission from botulinum toxin. J Physiol. 1977 Mar;265(3):809–820. doi: 10.1113/jphysiol.1977.sp011745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes-Anderson E., Raviola G. Degeneration and regeneration of autonomic nerve endings in the anterior part of rhesus monkey ciliary muscle. J Neurocytol. 1978 Oct;7(5):583–600. doi: 10.1007/BF01260891. [DOI] [PubMed] [Google Scholar]
- Tuffery A. R. Growth and degeneration of motor end-plates in normal cat hind limb muscles. J Anat. 1971 Nov;110(Pt 2):221–247. [PMC free article] [PubMed] [Google Scholar]
- Vyskocil F., Magazanik L. G. Dual end-plate potentials at the single neuromuscular junction of the adult frog. Pflugers Arch. 1977 Apr 25;368(3):271–273. doi: 10.1007/BF00585207. [DOI] [PubMed] [Google Scholar]
- Wernig A., Pécot-Dechavassine M., Stover H. Sprouting and regression of the nerve at the frog neuromuscular junction in normal conditions and after prolonged paralysis with curare. J Neurocytol. 1980 Jun;9(3):278–303. doi: 10.1007/BF01181538. [DOI] [PubMed] [Google Scholar]