Abstract
1. The stimulus-response function of the red rods in the retina of the common frog (Rana temporaria) was determined in different adaptational states by measuring aspartate-isolated receptor responses. 2. Flash stimuli, background adaptations and bleaches were delivered through the same optical channel forming an oblique light-beam striking the receptor side of the isolated and flat-mounted retina at an angle of 10 degrees. 3. When the light was blue-green and optimally polarized the absorbance of the receptor layer was about 2, from which follows that 70-80% of the light was absorbed in the distal third of the rod outer segments, i.e. the exposure was local. Homogeneous exposures of the whole rod outer segments were obtained with orange and red lights. 4. Combinations of homogeneous and local stimuli with homogeneous and local adaptations were used to investigate the longitudinal spread of background, intermediate and opsin adaptation, i.e. the sensitivity-reducing effect of a background light, and the transient and permanent sensitivity losses following a bleach isomerizing 3.5-26% (usually 10%) of the rhodopsin in the retina. 5. The results obtained were related to predictions based both on the assumption that the adaptation effects spread longitudinally within the rod outer segments and the assumption that they are strictly confined to the disks absorbing the adapting lights. 6. These comparisons reveal that all three types of adaptation spread longitudinally. It is for instance clear that the sensitivity loss observed with homogeneous stimuli and local adaptation (as compared to homogeneous adaptation) is larger than that predicted by the non-spreading hypothesis. 7. The longitudinal spread of background adaptation is largely finished within 10 sec after turning on the background light, while an efficient spread of the intermediate adaptation effect may require minutes. 8. A background light decreasing the sensitivity by about one log unit decreases the time from flash to response maximum from 5 to 1 sec (small responses). Corresponding opsin adaptation effects are accompanied by less dramatic changes in response kinetics. 9. Independent of adaptation type - homogeneous or local, background, intermediate or opsin - it was found that local stimuli are less efficient that homogeneous stimuli in light-adapted retinae. This effect can be explained assuming that the sensitivity-reducing effects are pronounced in the distal than in the proximal parts of the rod outer segments. 10. The opsin adaptation effect following 10% local bleaches decreases the sensitivity to both homogeneous and local stimuli 2-3 times more than corresponding homogeneous bleaches. This means that the strength of the opsin effect is not related to the average percentage bleached but to the fraction bleached in the distal part of the rod, or generally to the fraction bleached in the most affected region. 11...
Full text
PDF


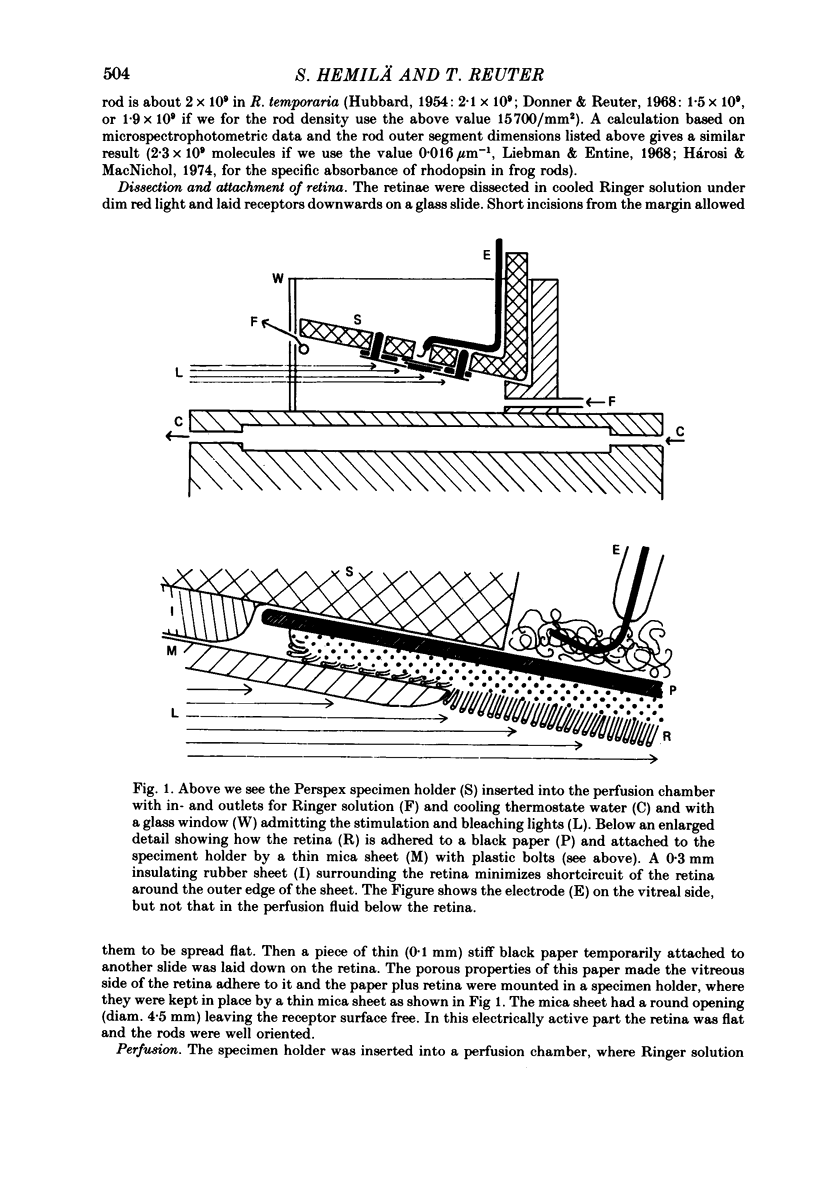




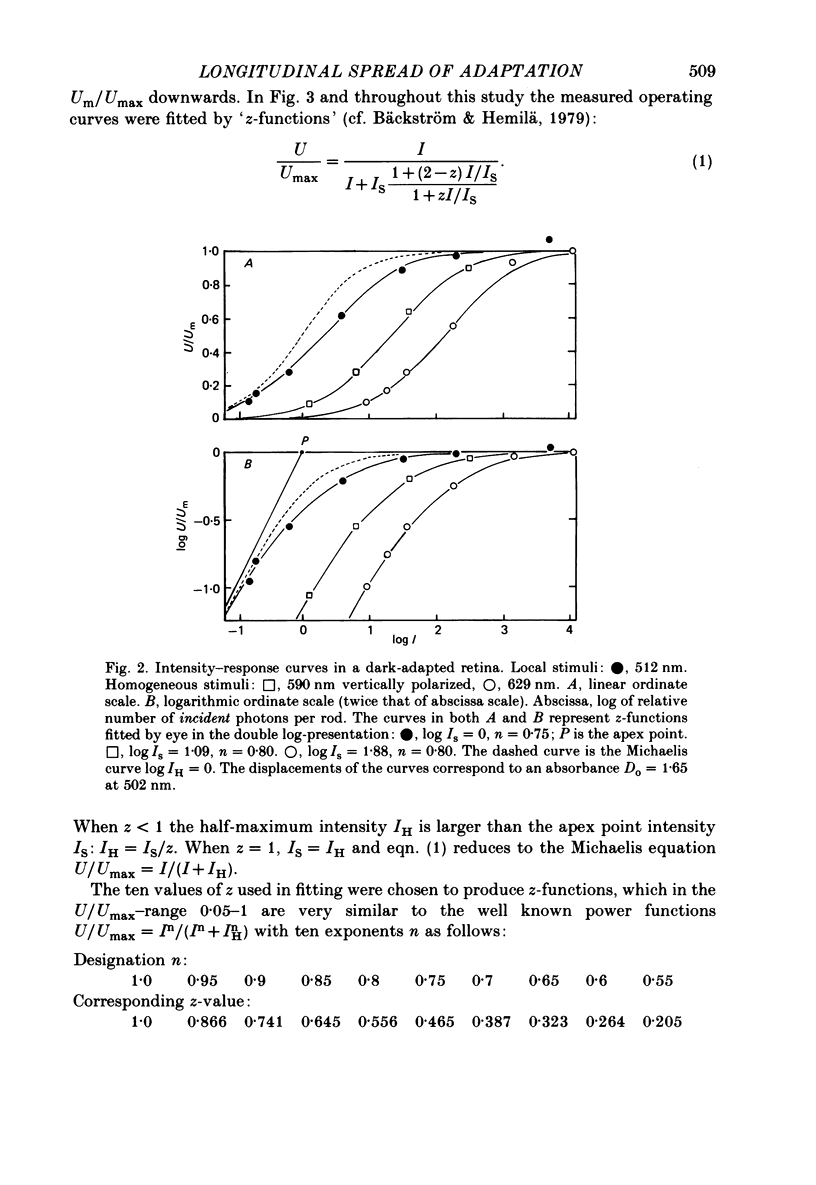

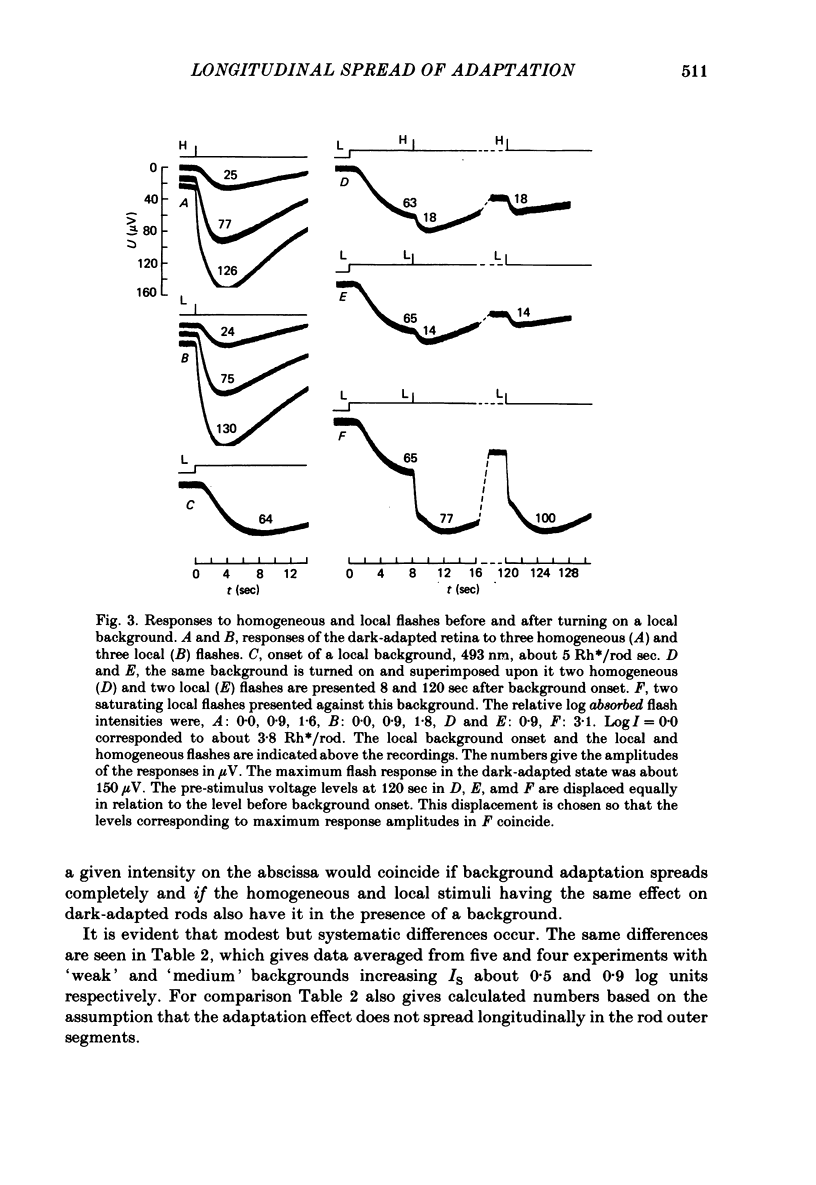
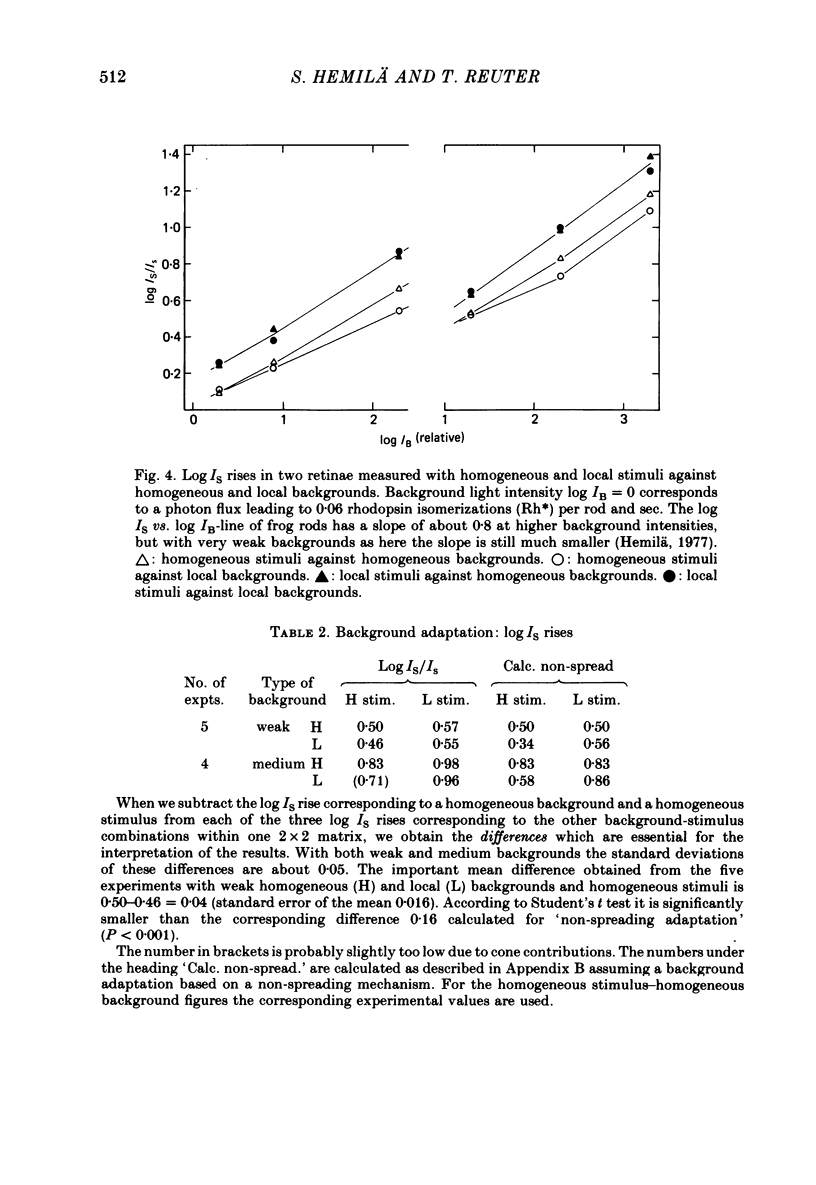











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastian B. L., Fain G. L. Light adaptation in toad rods: requirement for an internal messenger which is not calcium. J Physiol. 1979 Dec;297(0):493–520. doi: 10.1113/jphysiol.1979.sp013053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Bäckström A. C., Hemilä S. O. Dark-adaptation in frog rods: changes in the stimulus-response function. J Physiol. 1979 Feb;287:107–125. doi: 10.1113/jphysiol.1979.sp012649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Pasino E., Torre V. Electrical responses of rods in the retina of Bufo marinus. J Physiol. 1977 May;267(1):17–51. doi: 10.1113/jphysiol.1977.sp011799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONNER K. O., RUSHTON W. A. Retinal stimulation by light substitution. J Physiol. 1959 Dec;149:288–302. doi: 10.1113/jphysiol.1959.sp006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey M. M., Davis P. K., Blasie J. K., Barr L. Localization of rhodopsin antibody in the retina of the frog. J Mol Biol. 1969 Feb 14;39(3):395–405. doi: 10.1016/0022-2836(69)90134-x. [DOI] [PubMed] [Google Scholar]
- Donner K. O., Hemilä S. O. Dark-adaptation of the aspartate-isolated rod receptor potential of the frog retina: threshold measurements. J Physiol. 1979 Feb;287:93–106. doi: 10.1113/jphysiol.1979.sp012648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner K. O., Hemilä S. Excitation and adaptation in the vertebrate rod photoreceptor. Med Biol. 1978 Apr;56(2):52–63. [PubMed] [Google Scholar]
- Donner K. O., Hemilä S., Reuter T. Bleaching and background adaptation in frog rods. Vision Res. 1979;19(4):399–400. doi: 10.1016/0042-6989(79)90103-2. [DOI] [PubMed] [Google Scholar]
- Donner K. O., Reuter T. The dark-adaptation of single units in the frog's retina and its relation to the regeneration of rhodopsin. Vision Res. 1965 Dec;5(11):615–632. doi: 10.1016/0042-6989(65)90035-0. [DOI] [PubMed] [Google Scholar]
- Donner K. O., Reuter T. The photoproducts of rhodopsin in the isolated retina of the frog. Vision Res. 1969 Jul;9(7):815–847. doi: 10.1016/0042-6989(69)90017-0. [DOI] [PubMed] [Google Scholar]
- Donner K. O., Reuter T. Visual adaptation of the rhodopsin rods in the frogs retina. J Physiol. 1968 Nov;199(1):59–87. doi: 10.1113/jphysiol.1968.sp008639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbretson G. A., Witkovsky P. Rod sensitivity and visual pigment concentration in Xenopus. J Gen Physiol. 1978 Dec;72(6):801–819. doi: 10.1085/jgp.72.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govardovskii V. I. The sites of generation of early and late receptor potentials in rods. Vision Res. 1975 Aug-Sep;15:973–980. doi: 10.1016/0042-6989(75)90239-4. [DOI] [PubMed] [Google Scholar]
- HUBBARD R. The molecular weight of rhodopsin and the nature of the rhodopsin-digitonin complex. J Gen Physiol. 1954 Jan 20;37(3):381–399. doi: 10.1085/jgp.37.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hemilä S. An analysis of rod outer segment adaptation based on a simple equivalent circuit. Biophys Struct Mech. 1978 Apr 13;4(2):115–128. doi: 10.1007/BF00539226. [DOI] [PubMed] [Google Scholar]
- Hemilä S. Background adaptation in the rods of the frog's retina. J Physiol. 1977 Mar;265(3):721–741. doi: 10.1113/jphysiol.1977.sp011740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hárosi F. I., MacNichol E. F., Jr Dichroic microspectrophotometer: a computer-assisted, rapid, wavelength-scanning photometer for measuring linear dichroism in single cells. J Opt Soc Am. 1974 Jul;64(7):903–918. doi: 10.1364/josa.64.000903. [DOI] [PubMed] [Google Scholar]
- Jagger W. S. Local stimulation and local adaptation of single isolated frog rod outer segments. Vision Res. 1979;19(4):381–384. doi: 10.1016/0042-6989(79)90098-1. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J., Dowling J. E. Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol. 1975 Nov;66(5):617–648. doi: 10.1085/jgp.66.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock J. H., Reuter T. Retinal ganglion cells in the crucian carp (Carassius carassius). I. Size and number of somata in eyes of different size. J Comp Neurol. 1978 Jun 1;179(3):535–547. doi: 10.1002/cne.901790306. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Visual pigments of frog and tadpole (Rana pipiens). Vision Res. 1968 Jul;8(7):761–775. doi: 10.1016/0042-6989(68)90128-4. [DOI] [PubMed] [Google Scholar]
- McNaughton P. A., Yau K. W., Lamb T. D. Spread of activation and desensitisation in rod outer segments. Nature. 1980 Jan 3;283(5742):85–87. doi: 10.1038/283085a0. [DOI] [PubMed] [Google Scholar]
- NILSSON S. E. THE ULTRASTRUCTURE OF THE RECEPTOR OUTER SEGMENTS IN THE RETINA OF THE LEOPARD FROG (RANA PIPIENS). J Ultrastruct Res. 1965 Feb;12:207–231. doi: 10.1016/s0022-5320(65)80016-8. [DOI] [PubMed] [Google Scholar]
- Sillman A. J., Ito H., Tomita T. Studies on the mass receptor potential of the isolated frog retina. II. On the basis of the ionic mechanism. Vision Res. 1969 Dec;9(12):1443–1451. doi: 10.1016/0042-6989(69)90060-1. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrical activity of vertebrate photoreceptors. Q Rev Biophys. 1970 May;3(2):179–222. doi: 10.1017/s0033583500004571. [DOI] [PubMed] [Google Scholar]


